THE PYCNOGONIDA
THE pycnogonids are strange-looking marine animals with small bodies and long, sprawling legs (fig. 15 A, C). Most of them measure a few centimeters across the outspread appendages, but some only 3 or 4 mm., while a deep-sea species has a spread of 50 cm., about 18 inches. The animals inhabit all parts of the ocean, but are most numerous in arctic and antarctic regions; they live chiefly along the shore, though a few species are deepwater inhabitants. Because of the number and length of their legs, the pycnogonids are known as “sea spiders.” The name Pycnogonida refers to the crowded condition of the numerous “knees” when the legs are bent upward on their bases; but another group name is Pantopoda, which presumably expresses the fact that when the animals are crawling they appear to be “all legs.” The number of appendages varies from four to eight or even nine pairs in different species.
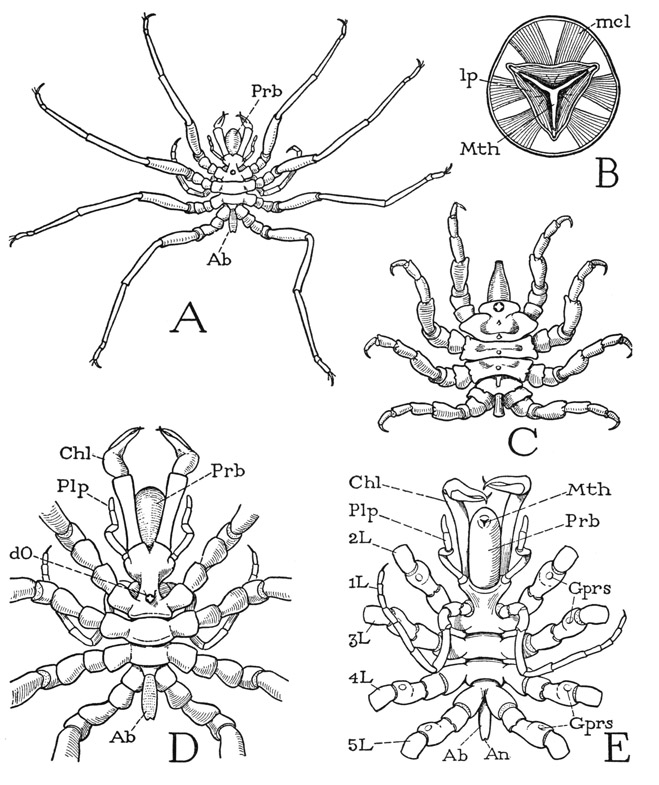
As an example for a study of the general structure of a pycnogonid we may take one named Nymphon hirtipes Bell (fig. 15 A), a common subtidal species of the northern Atlantic coast of North America, having seven pairs of appendages. The trunk of the animal is divided into a partially segmented, limb-bearing prosoma (D, E), from which projects forward a large proboscis (Prb), and a very small, simple, unsegmented opisthosoma, or abdomen (Ab). The prosoma includes an anterior unsegmented section bearing the first four pairs of limbs and, on the posterior part of its dorsal surface (D), a tubercle with four very small eyes (dO); its posterior part is composed of three distinct segments, each of which carries a pair of legs. All the limbs are supported on large lateral lobes of the body. The minute abdomen (Ah) is a slender tube projecting posteriorly from between the bases of the last pair of legs and bears the anus at its extremity (E, An).
Fig. 15. Pycnogonida (Pantapoda).
A, Nymphon hirtipes Bell, female, dorsal. B, same, cross section of proboscis just behind the mouth. C, Pycnogonum littorale Ström., female, dorsal. D, Nymphon hirtipes Bell, female, body and bases of legs, dorsal. E, same, ventral.
Ab, abdomen; An, anus; Chi, chelicera; dO, dorsal ocelli; Gprs, gonopores; L, leg; lp, lip; mcl, muscle; Mth, mouth; Plp, palp; Prb, proboscis.
The appendages include a pair of relatively large, three-segmented chelicerae, or chelifores (fig. 15 D, E, Chl), embracing the proboscis, a pair of short, slender, five-segmented appendages, known as the palpi (Plp), closely associated with the bases of the chelicerae, and five pairs of many-jointed legs (E, IL–5L). The first legs (1L), however, differ from the others in their extreme slenderness and their ventral position. These appendages are termed the ovigerous legs because the male uses them for carrying the eggs (fig. 16, H, I), which are loaded upon him by the female at the time of mating. They are present in both sexes of the species here described, but in the females of some families they are absent. The following four pairs of appendages are the walking legs (fig. 15 E, 2L–5L), and are all practically alike in size and structure. The segmentation of the legs will be discussed later. Inasmuch as there are seven pairs of appendages on the prosoma, this part of the body must include at least seven primitive segments. The anterior section bearing the chelicerae, the palps, the ovigerous legs, and the first pair of walking legs, therefore, is a composite tagma of four segments, and only the posterior three prosomatic segments remain ununited.
The huge proboscis is a characteristic feature of the pycnogonids, though it varies in relative size in different species; in Pycnogonum, for example (fig. 15 C), it is small compared with the proboscis of Nymphon (A, D, E, Prb), and in some forms it is almost as large as, or even larger than, the body itself. The mouth is situated on the anterior end of the proboscis (E, Mth) and is enclosed between three small lips, one dorsal, the other two ventrolateral, so that when the mouth is closed (B, E), the aperture is Y-shaped, but becomes triangular when the mouth is opened. The mouth leads directly into the cavity of a large sac occupying the entire length of the proboscis. In cross section the sac is triangular (B), its three sides corresponding with the three lips (lp) of the mouth (Mth). In the grooves between the sides are slender rods proceeding inward from the mouth angles. Each lip is provided with a pair of large muscles (mcl) arising behind the mouth on the walls of the proboscis, and throughout its length the walls of the sac are covered with radiating bundles of dilator muscle fibers with their peripheral attachments on the wall of the proboscis. The inner sac of the pycnogonid proboscis thus much resembles the sucking “pharynx” of an arachnid, but it has no constrictor muscles.
The lips of the mouth are strongly sclerotized along their edges and are fringed with very small hairs; in Nymphon hirtipes they have no armature other than two minute teeth just within the apex of each lip. As noted by Dohrn (1881) for the pycnogonids in general, the lips have no muscular closing apparatus; the mouth apparently shuts by its own elasticity. The inner sac also, though clearly dilatable by its radial muscles, has no antagonistic compressor muscles. The walls of the sac are smooth on their distal parts and devoid of spines, but on the inner half of each section are several broad, overlapping brushes of thickly set, extremely delicate hairs directed toward the mouth. The proboscis sac of Nymphon hirtipes, therefore, appears to be merely a sucking apparatus, and certainly can have no mechanical action on the ingested food. The brushes evidently serve as filters guarding the entrance to the oesophagus. The structure of the pycnogonid proboscis and its variations in different species have been fully described by Dohrn (1881). The distal end of the inner sac is surrounded by a ganglionated nerve ring, connected by a median dorsal nerve with the brain and by a pair of ventrolateral nerves with the suboesophageal ganglion. From the ring a nerve then proceeds posteriorly on the wall of each section of the sack, and the three are united by circular commissures, from which the proboscis muscles are innervated (see Wirén, 1918; Hanström, 1928). The proboscis sac leads into a short oesophagus, and the oesophagus discharges into a mesenteron stomach, from which are given off tubular diverticula that extend into all the walking legs and into some of the other appendages.
The pycnogonids feed on soft animals, particularly on coelenterate polyps. Their methods of feeding have been described by Cole (1906) and by Prell (1910). An individual of Nymphon feeding on the hydrozoan Campanularia, as observed by Prell, when it happens on a hydranth tentacle, grasps the stalk of the latter with one of its chelicerae and brings the hydrotheca against its mouth. A sucking action of the proboscis now begins, and the tentacle of the hydranth slowly disappears into the mouth of the pycnogonid. Finally the last cellular connection is broken, and the empty hydrotheca is cast away. The ingested material, Prell says, forms a conical mass within the proboscis, which in a short time, by the ceaseless action of the straining apparatus, is reduced to plasmatic lumps, which go into the oesophagus. The description by Cole of an Anoplodactylus feeding on Eudendrium is essentially the same, except that Cole says the hydranth is broken off and forced into the mouth by the chelicerae, or sometimes broken up by the pincers and the pieces then apparently sucked into the mouth. A species of Phoxichilidium is recorded by Prell as feeding on a variety of hydrozoans, on the scyphozoan Lucernaria, and also on Bryozoa. In attacking Lucernaria it grasps the stalk of a tentacle group with one of its chelae, cuts it off, and brings the severed end to its mouth, whereupon the sucking action of the proboscis begins. Pycnogonum littorale, according to Prell, feeds on sea anemones (Actinozoa). Pycnogonum has no chelicerae (fig. 15 C), but it clings to the anemone by its feet and thrusts its proboscis into the body of the victim. The end of the proboscis is abruptly narrowed, but the mouth has no armature other than the three triangular, hard-edged, jawlike lips, which evidently are of sufficient strength to cut the skin of the anemone.
The food material ingested by the Pycnogonida, according to Schlottke (1933), contains no solid particles or remnants of tissue, indicating that it must be at once subjected to enzyme action, since it could not be reduced to this condition by the straining apparatus in the proboscis. Schlottke observes that the mesenteron discharges secretion into the lumen shortly before the beginning of food intake. The liquefied food is then absorbed and digested within cells of the mesenteron walls and diverticula; the digestive cells, finally containing only waste products, are cast off from the epithelium and discharged through the intestine. The whole process of feeding and digestion in the Pycnogonida is clearly very similar to that in Arachnida.
The appendages of the Pycnogonida are of special interest because of their numerous segments, their differentiated structure, and particularly their variable number in related species, a thing hardly to be expected in a group of animals otherwise so nearly alike. The large, three-segmented, chelate first appendages of Nymphon (fig. 16 C, Chl) and of most other genera appear to be identical with the chelicerae of Limulus and the arachnids, and they are said to be likewise innervated from the tritocerebral ganglia of the central nervous system. The chelicerae arise from anterior lobes of the body at the sides of the base of the proboscis. In a few families the chelicerae are absent, as in Pycnogonum (fig. 15 C), or, if present, they may be nonchelate. The second appendages, or palpi (fig. 15 D, E, Plp), may very well correspond with the pedipalps of the arachnids. In Nymphon hirtipes they are short, slender appendages of five segments each, arising from small body lobes at the sides of the cheliceral lobes (fig. 16 C, Plp). In other forms the palpi have a variable number of segments, and in many genera they are much reduced in size, or are absent as in Pycnogonum. The third appendages, or ovigerous legs (fig. 15 E, IL), arise ventrally on the anterior section of the body close in front of the bases of the first walking legs. They are well developed in both sexes of Nymphon hirtipes, though they differ somewhat in the male and the female; they appear to have 11 segments, counting the terminal claw, but their structure can be better described after a study of the walking legs. The presence or absence of the first three appendages, and their variable character in the different families and principal genera of the Pycnogonida are shown in tabular form by Hedgpeth (1947).
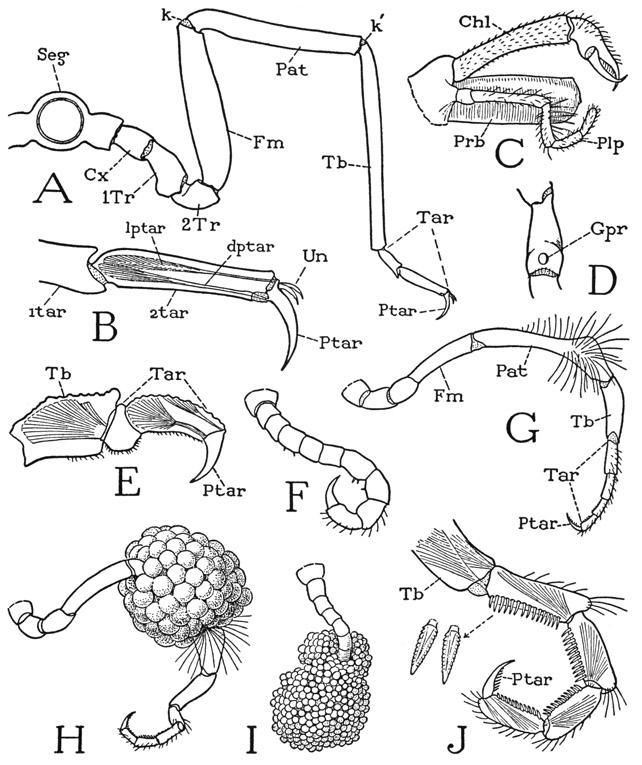
The four pairs of long walking legs do not differ essentially from one another in length, shape, or segmentation. Each leg (fig. 16 A) has nine apparent segments, but a study of the musculature will show that the two parts between the tibia (Tb) and the pretarsus (Ptar) are tarsal subsegments (Tar). The pycnogonid leg strikingly resembles an arachnid leg in having a patellar segment (Pat) that takes a horizontal position between the femur and the tibia, so that the leg might be said to have a double knee (k, k’). In the basal part of each leg, proximal to the femur (Fm), are three short segments that would appear to be the coxa (Cx), a first trochanter (1Tr), and a prefemur, or second trochanter (2Tr). The first segment has a horizontal hinge on the supporting body lobe, instead of the more usual vertical hinge of the coxa on the body; the second segment has a vertical hinge on the first, but the next two joints have horizontal hinges. Among the Arachnida three similar basal segments are present in some of the legs of Solpugida. The pycnogonid leg as a whole turns up and down in a vertical plane on its basal hinge with the body, and its only point of anterior and posterior movement is at the joint between the first and second segments. The musculature of each of the segments of the leg is appropriate to the movement at the joint with the segment proximal to it, the second segment having anterior and posterior muscles, while all the others have dorsal and ventral muscles, except the tarsus, which in the walking legs has only a ventral muscle. The movements of the legs of Pycnogonida have been described by Cole (1901) and by Prell (1910), and it is easily seen that the action of the legs at the joints is precisely adapted to the way the pycnogonids move their legs, which is principally up and down both in walking and in swimming.
The apical segment of the pycnogonid leg is a decurved claw (fig. 16 A, Ptar), which is clearly shown to be the pretarsus by the presence of a levator and a depressor muscle inserted on its base (B). In Nymphon hirtipes two small accessory claws (B, Un) arise from the base of the pretarsus, but in other species there may be only a single accessory claw or none. Intervening between the pretarsus and the end of the tibia are two segmentlike divisions of the leg, the first of which is usually termed the “propodus” and the second the "tarsus.” Since no muscles connect these two parts, however, the latter are simply subdivisions of the tarsus (A, Tar). Attached on the base of the first tarsomere is a long depressor muscle of the tarsus arising in the tibia. The same tarsal structure and musculature are seen in the much shorter leg of Pycnogonum (E). The tibia and the patella have each two antagonistic muscles, a levator and a depressor. The simple musculature of the pycnogonid leg has little resemblance to the leg musculature of Limulus.
The first legs, or ovigers as they are called, in Nymphon hirtipes differ somewhat between the male and the female. In the female they are uniformly slender with no particular differentiation of the segments (fig. 15 E, 1L). In the male (fig. 16 G) the patella (Pat) is disproportionately long and abruptly thickened at its distal end, where it bears a brush of long stiff hairs on the outer surface. In an egg-carrying leg (H) it is seen that the egg mass surrounds the slender part of the patella, and is held here by the distal enlargement of the segment and the bristlelike hairs. Between the tibia and the pretarsus of the ovigerous leg in each sex of Nymphon are four short divisions of the limb (G, Tar) in what appears to correspond with the two-part tarsal section of a walking leg (A, Tar). An examination of these four parts of the ovigerous leg, however, reveals the unexpected fact that they are individually musculated (J), each having a flexor muscle inserted on its base arising in the part proximal to it, while the first has two muscles arising in the tibia. The presence of intratarsal muscles in the ovigerous legs of the pycnogonids was noted by Börner (1903), but in no other arthropods are tarsal subdivisions known to be equipped individually with muscles. A segmentation of a different type appears to be present in the ovigerous leg of Anoplodactylus lentus. The oviger of this species consists of only seven segments, the terminal claw segment being absent, and at each movable joint there are two antagonistic muscles, suggesting that the terminal segment alone is the tarsus. In Pycnogonum littorale ovigerous legs are present only in the male; they are relatively very small (F) and the segments are but little differentiated in size, but the number of them is the same as in Nymphon. An egg-bearing leg of Pycnogonum (I) has the claw-bearing tarsal segments simply hooked into the egg mass. In Nymphon hirtipes the ventral sides of the tarsal segments and the pretarsal claw are armed with combs of small denticulate spines (J), and these segments are usually strongly flexed. It is shown by Prell (1910) that the tarsi of the ovigerous legs are used to clean the other legs.
Fig. 16. Pycnogonida (Pantapoda).
A, Nymphon hirtipes Bell, female, section of body with leg. B, same, tarsus and pretarsus of a walking leg, showing pretarsal muscles. C, same, proboscis, chelicera, and palp of right side. D, same, female, first trochanter of a walking leg, with opening of oviduct on ventral surface. E, Pycnogonum littorale Ström., tarsus and pretarsus of a walking leg, showing muscles. F, same, ovigerous leg of male. G, Nymphon hirtipes Bell, ovigerous leg of male. H, same, ovigerous leg of male bearing eggs. I, Pycnogonum littorale Ström., ovigerous leg of male bearing eggs. J, Nymphon hirtipes Bell, distal segments of ovigerous leg of male, showing tarsal segments individually musculated.
Cx, coxa; dptar, depressor muscle of pretarsus; Fm, femur; Gpr, gonopore; k, k’, double knee bend of leg; lptar, levator muscle of pretarsus; Pat, patella; Ptar, pretarsus; Seg, body segment; Tar, tarsus; 1tar, first tarsal subsegment; 2tar, second tarsal subsegment; Tb, tibia; 1Tr, first trochanter; 2Tr, second trochanter; Un, ungues.
The exit apertures of the reproductive system of the pycnogonids are on the second segments of the walking legs, generally but not always on the undersurfaces (fig. 16 D, Gpr). In the female there is usually an opening on each leg (fig. 15 E, Gprs), but in the male the openings may be fewer, sometimes limited to a single pair on the last legs; in Nymphon hirtipes the male has apertures on the last two pairs of legs. The testes and the ovaries lie in the body above the alimentary canal, but they send branches into the walking legs; in a gravid female the femora may be seen to be full of developing eggs. The pycnogonids are unique among the arthropods in the possession of multiple genital openings, but it is not anomalous that the exits should be on the bases of the legs; this undoubtedly was their position in the primitive arthropods, since the genital ducts are to be traced back to coelomic ducts opening at the bases of the appendages, as in the modern Onychophora. In Limulus the genital apertures are on the opercular appendages of the eighth body segment, and in many Crustacea and most Diplopoda they are on the coxae of a specific pair of legs. The position of the gonopores in the Pycnogonida, therefore, is exceptional only in that it is on the second leg segment instead of the first.
It is impossible to arrive at any positive conclusion concerning the relationships of the Pycnogonida, except that the animals are arthropods. The absence of antennae and the presence of three-segmented, chelate first appendages might seem in themselves sufficient evidence for including the pycnogonids with the merostomes and arachnids in the large group of Chelicerata, but, as seen in the arachnids and crustaceans, chelae may be independently developed on almost any of the limbs, and in some other arthropods antennae are absent. However, the pycnogonids have the arachnid type of leg, and the eyes are said to have the “inverted” structure characteristic of the median eyes of arachnids (Wirén, 1918). A common feature of the Xiphosurida, Arachnida, and Pycnogonida is the branching of the stomach into radiating, tubular diverticula, in the walls of which, according to Schlottke (1933), the final phase of digestion takes place intracellularly. The inner sac of the proboscis that opens into the oesophagus resembles the sucking organ of the arachnids, and the proboscis itself might be comparable to the snout of the primitive Palpigradi among the Arachnida (fig. 23 D).
The pycnogonids, of course, differ from the other chelicerates, except Solpudiga, in that the leg-bearing part of the body is partly segmented and that the opisthosoma is reduced to a diminutive abdomen. The unsegmented anterior part of the body carrying the first four pairs of limbs might be likened to the “head” of a trilobite with its four pairs of legs, but the idea of a trilobite relationship for the pycnogonids is hardly to be entertained. All the pycnogonids have at least four pairs of walking legs, which gives them a spiderlike appearance, but since there are usually three pairs of appendages in front of the walking legs, the pycnogonids in general have seven pairs of limbs, and hence are more comparable to Limulus in the number of appendages, since the fourth pair of pycnogonid walking legs would correspond with the chilaria of the xiphosurids. In three families of the Pycnogonida, however, there are forms having five pairs of walking legs, and in one of these families a six-legged species is known. To keep the comparison with Limulus, then, it would seem that we should have to regard the extra legs as representing the first two pairs of opisthosomatic appendages; but, since the last leg-bearing segment and the abdomen retain the same structure regardless of the number of legs, it is probable that the extra pairs, when present, are interpolations somewhere between the other legs. It has been shown by Hedgpeth (1947) that the number of legs in the Pycnogonida can have no phylogenetic or taxonomic significance, since the five-legged forms occur in widely separated families and are closely related to four-legged forms in these same families, and the single species known to have six pairs of walking legs belongs to a family including four-legged and five-legged forms.
In conclusion, then, we can only repeat what was said at the beginning, that the pycnogonids are queer animals. But still, we can feel safe in claiming them for arthropods, and probably as a branch of the Chelicerata having more arachnid than merostome characters. Particularly arachnoid is their method of feeding and their sucking apparatus for the ingestion of food. For a full account of the pycnogonids the student should consult Heifer and Schlottke (1935).