THE ARACHNIDA
THE arachnids are more easily recognized than defined. They have so many features in common with Limulus that some zoologists have classed Limulus in the Arachnida. The essential differences between the Xiphosurida and the Arachnida are in the feeding organs and the organs of respiration. The arachnids feed on liquids extracted from their prey, which are ingested by a pharyngeal sucking pump; the xiphosurids feed on solid food, which is ground up in a proventricular grist mill. The arachnids are terrestrial and breathe by means of lungs or tracheae; the xiphosurids, being aquatic, have abdominal gills, and theoretical attempts to derive the arachnid lungs from gills are not convincing.
The most primitive of modern arachnids, the Palpigradi, are more generalized than Limulus. The Xiphosurida and the Arachnida, therefore, are two branches of the subphylum Chelicerata, but their common ancestors are not known. While there are paleontological reasons for believing that the xiphosurids and the trilobites had a common progenitor, the actual origin of the arachnids is obscure. However, as was noted in the last chapter, the pycnogonids have some surprisingly arachnoid characters. The scorpions have a superficial resemblance to the Eurypterida, but the scorpion, as compared with the Palpigradi, is not a primitive arachnid. However, it is not an object of the present text to discuss theoretical arthropod phylogeny. The student may learn the essentials of arachnid anatomy from a study of the scorpion, the spiders, and a tick, which are the principal subjects of this chapter.
THE SCORPION
The scorpion in appearance (fig. 17 A) is a highly distinctive arachnid that could not possibly be mistaken for any other member of the class; the combination of large, chelate pedipalps with a sting at the end of a segmented tail alone proclaims the animal to be a true scorpion. The so-called whip scorpion (fig. 23 E) and the pseudoscorpion have no sting. About 600 species of scorpions are known, most of them two to four inches in length, but there is one only half an inch long, while the huge African Pandinus attains a length of seven inches. Scorpions are widely distributed throughout the tropical parts of the earth and in most of the warmer regions of the temperate zones; 22 species occur in the United States. For a general account of the habits and modes of life of scorpions the reader is referred to the article “Scorpion” by Petrunkevitch in the Encyclopaedia Britannica (1947).
General Structure of a Scorpion
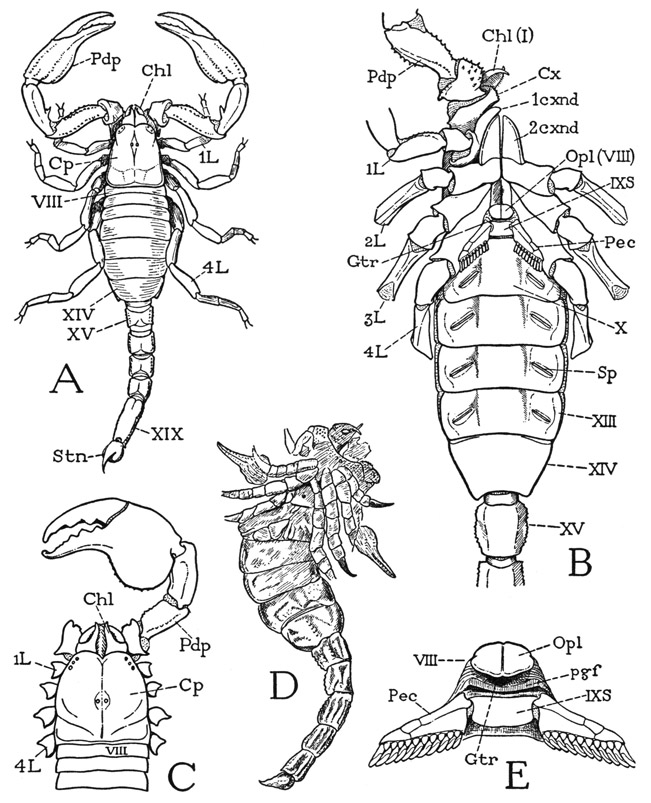
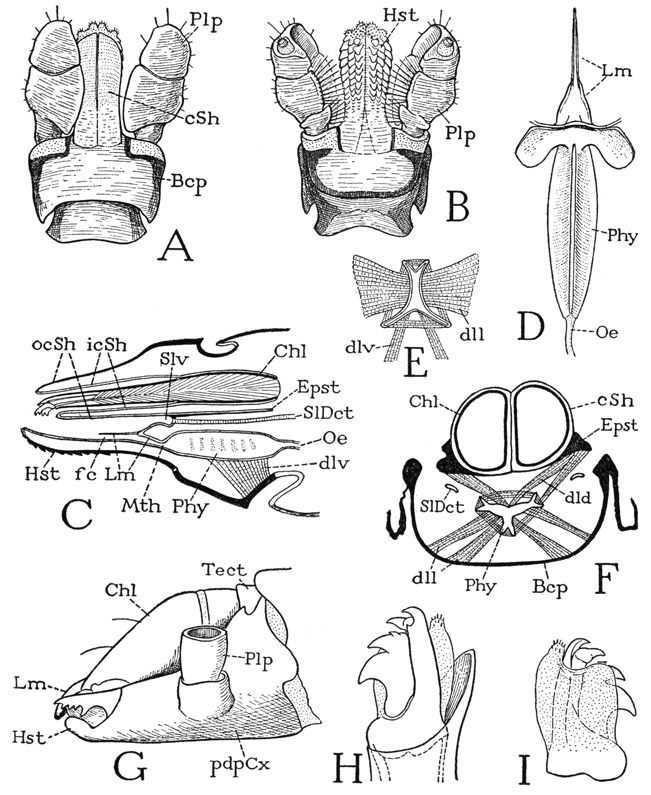
A scorpion at first glance (fig. 17 A) appears to have an elongate oval body, supported on four pairs of legs, and a thick, jointed tail bearing the sting at its extremity. The body, however, is divided into an unsegmented anterior part, which alone bears the appendages, and a larger segmented posterior part; the tail is a slender extension of the body, consisting of five segments, with the anus in the last segment. Morphologically considered, then, the trunk of the scorpion includes a prosoma, covered by an unsegmented plate, or carapace, and a segmented opisthosoma, or abdomen, which is differentiated into an anterior mesosoma, or preabdomen, and a posterior metasoma, or postabdomen, which is the tail. The prosoma bears the usual arachnid appendages, which are the chelicerae (A, B, Chi), the pedipalps (Pdp), and four pairs of legs (1L–4L); it therefore includes six primary postoral somites, and is thus comparable with the prosoma of Limulus, except that it lacks the chilaria and the corresponding seventh segment. The seventh body segment of the scorpion, in fact, is known to be suppressed during embryonic development, so that the first segment of the opisthosoma in the adult is the eighth. The mesosoma contains seven segments, which are segments VIII–XIV, and the tail has five segments (XV–XIX), not including the terminal sting. The adult scorpion, therefore, has 18 postoral segments in all, which is the maximum number of segments possessed by any other arachnid, but if we count the suppressed seventh segment, it has 19 segments.
Fig. 17. Arachnida—Scorpionida.
A, Chactas vanbenedeni Gervais, Chactidae (2 lateral eyes on each side), Colombia. B, Pandinus sp., Scorpionidae, Congo, ventral surface. C, same, dorsal (3 lateral eyes on each side). D, Palaeophonus hunteri Pocock, Scottish Silurian scorpion, 35.5 mm. long (from Pocock, 1901). E, Pandinus sp., genital region of segment VIII, and pectines of segment IX, ventral.
For explanation of lettering see pages 126–127.
Studies by different writers on the correlation of the nerve centers of the scorpion with the body segmentation have given somewhat different results. McClendon (1904) described 20 pairs of neuromeres, or primary segmental ganglia, of which the first pair forms the brain, and the other 19 the ventral nerve cord. According to Buxton (1917), however, there are only 18 neuromeres in the nerve cord, which is one less than the number of body segments. Both Buxton and Petrunkevitch (1949), therefore, suggest that the first tail segment is a secondary subdivision of the last mesosomatic segment. Kästner (1940), on the other hand, re-examining the subject in species of several genera, finds that there are 19 pairs of primary ganglia formed in the postoral nervous system, of which the cheliceral ganglia unite with the brain, and the ganglia of segment VII disappear along with the segment itself; in other words, there is at first a pair of ganglia for each of the primary 19 postoral segments. In the adult, however, the five postcheliceral ganglia of the prosoma and the first four persisting opisthosomatic ganglia unite in a large nerve mass, or suboesophageal ganglion, lying in the prosoma, while the ganglia of segments XII to XVII remain separate, though the first two are displaced forward, and the ganglia of segments XVIII and XIX unite to form a double last ganglion lying in segment XVIII.
The carapace of the prosoma (fig. 17 A, C, Cp) is widest behind, somewhat narrowed anteriorly. On it are situated three groups of small, simple eyes, there being a pair of median eyes, and two groups of more anterior lateral eyes, each with from two to five eyes according to the species. No arachnid has compound eyes. The front margin of the carapace projects as a free fold over the bases of the chelicerae (fig. 21 D). From the inner end of the under lamella, or doublure, of the fold, the membranous anterior wall of the body goes downward to the base of a large median lobe (Lm), which is the labrum. The labrum lies between the bases of the pedipalps (B), but the chelicerae arise entirely dorsal to the labrum. At the base of the labrum is an irregular sclerotization of the body wall (D, Epst) representing the epistome of other arachnids, which is usually a horizontal plate forming a bridge between the upper surfaces of the pedipalp coxae and supporting the labrum. Though the adult arachnid has no distinct head, the labrum, the epistome, and the eyebearing region of the carapace are derived from the cephalic lobe of the embryo. The lateral margins of the carapace are not extended beyond the leg bases (B), and a cross section of the prosoma of the scorpion (fig. 22 A), therefore, has quite a different shape from that of Limalus (fig. 7 A). The edges of the carapace are separated from the bases of the legs by narrow pleural folds of the integument (figs. 19 E, 22 A, Pl). The ventral surface of the prosoma is occupied almost entirely by the coxae of the legs (fig. 17 B), there being only a small median sternal plate between the posterior two pairs of coxae.
The fully segmented mesosoma, or preabdomen, is much broader than the prosoma. On the dorsum (fig. 17 A) each of its seven segments is covered by a distinct tergal plate. On the undersurface (B) there are likewise seven segmental divisions, but the sternal plates are not all so simple or uniform in size as the tergal plates. The venter of the first segment (VIII) is wedged between the anterior ends of the last leg coxae, where it is greatly reduced in size and has no connection with the tergum of its segment. On it, however, is situated the genital opening, or gonotreme, in each sex (B, E, Gtr), which is covered by a small plate or pair of plates forming an operculum (Opl), and is bordered behind by a transverse postgenital fold (E, pgf) of the integument. The second opisthosomatic sternum (B, IXS) is a small quadrate plate bearing a pair of comblike appendages known as the pectines (Pec), which will be described in connection with the other appendages. This sternum also is separated from its tergum, except for narrow pleural folds that run along the posterior margins of the hind coxae. The following five mesosomatic sterna are broad plates united laterally with the corresponding terga by infolded pleural membranes (fig. 22 B, Pl) that allow a considerable dorsoventral expansion of the abdomen. The tergal and sternal margins of the last preabdominal segment, however, come together posteriorly and are united at the posterior angles of the segment. On the lateral areas of sterna X to XIII are oblique slits (fig. 17 B, Sp), which are the apertures, or spiracles, of the internal respiratory organs known as book lungs, to be described later.
The five segments of the metasoma, or tail, are simple rings, the tergal and sternal arcs being entirely confluent. In cross section (fig. 18 B) a tail segment has an octagonal shape with unequal sides. The segments have no specific articulations on each other, but they are strongly connected, and in such a manner that their principal movement is in a vertical plane, most freely in a dorsal direction (D), though lesser sidewise movements also are permitted. On the base of each segment are attached six muscles arising in the preceding segment, one dorsal (B, dmcl), four dorsolateral and ventrolateral (lmcls), and one ventral (vmcl). The basal segment has a corresponding but stronger musculature from the last mesosomatic segment. The dorsal flexion of the segments on one another allows the tail to be freely turned forward over the back of the scorpion and the inverted point of the sting to be thrust upward into the prey held in the jaws of the pedipalps. The tail is traversed by the long intestine (D, Rect), which opens ventrally in the end of the last segment (C, An) beneath the base of the sting. The segmentlike sting of the scorpion, as the tail spine of Limulus, is generally regarded as the telson, but in the mandibulate arthropods, especially in the Crustacea, the anus is situated on the apical telson, which fact might suggest that the sting-bearing segment of the scorpion is the true telson, and that the sting is a specialized appendage of it. On the other hand, the presence during embryonic development of a nerve center in the sting-bearing segment would appear to be conclusive evidence that this segment is a true somite and not the telson. Possibly, then, the anus has been transposed secondarily from the telson into the intersegmental membrane before it.
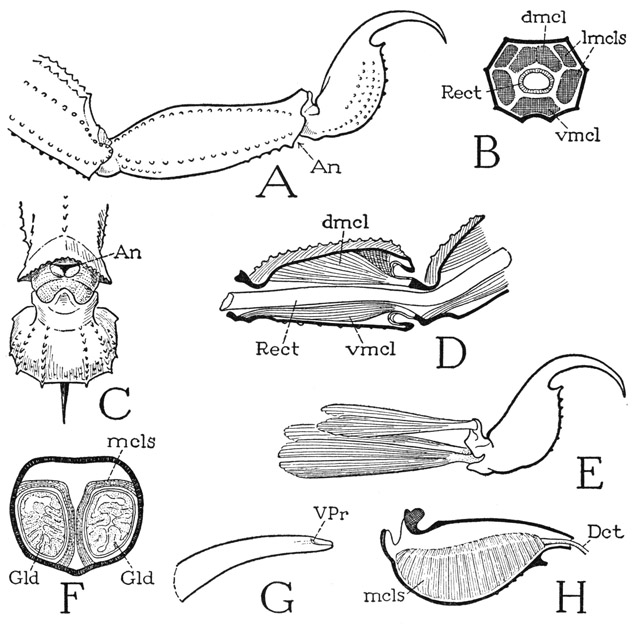
Fig. 18. Arachnida—Scorpionida. The postabdomen and the sting.
A, Centruroides sp., last two postabdominal segments and sting. B, same, cross section of a postabdominal segment, showing position of muscles. C, Pandinus sp., end of last postabdominal segment, with sting, ventral. D, Centruroides sp., longitudinal section of first postabdominal segment and base of second. E, same, the sting and its muscles. F, same, cross section through base of sting. G, same, terminal part of sting, showing aperture of left venom duct. H, same, right half of base of sting, mesal, showing muscles covering right venom gland, and exit duct.
For explanation of lettering see pages 126–127.
The sting of the scorpion (fig. 18 A) consists of a large, bulbous basal part and of a long, sharp, decurved distal spine that contains near its tip the outlets (G, VPr) of the venom glands in the bulb. The base of the organ is movably articulated on the end of the tail and is provided with four muscles (E) arising in the last tail segment, two dorsal and one ventrolateral on each side. In the reversed position of the tail, the smaller dorsal muscles depress the point of the sting, and the larger ventrolateral muscles give the upward thrust of the weapon into the body of the prey. Probably, acting as antagonists, the ventral muscles produce also lateral movements of the sting. The venom of the sting is secreted in two saclike glands contained in the swollen base of the organ (fig. 18 F, Gld). The glands have individual ducts opening separately near the point of sting through two lateral pores (G, VPr), from which grooves extend to the tip. Each gland is closely invested along its mesal and dorsal surfaces by a thick muscular sheath of several layers of semicircular fibers (F, H, mcls), attached dorsally above the gland on the outer wall of the containing capsule and ventrally on the lower wall. Contraction of the muscles evidently compresses the gland sacs against the rigid capsule walls and expels the venom through the ducts. The effect of the scorpion’s poison on insects captured for food is to kill them, but the effect on man is highly variable according to the species of scorpion. Some species produce symptoms no worse than those of a bee’s sting, while with others the results may be serious, and even fatal.
A cuticular endoskeleton is but little developed in the scorpion. Such as there is consists of a pair of epistomal apodemes, a small median apodeme of the prosomatic sternum (fig. 22 A, S), and apodemal extensions from the bases of the chelicerae and the coxae of the legs. Within the prosoma, however, is a noncuticular plate corresponding with the endosternum of Limulus, which gives attachment to numerous muscles but has no other connection with the body wall. The endosternum of the scorpion is much more complex than the simple endosternal plate of Limulus; its structure has been described and illustrated in detail by Lankester and Beck (in Lankester, Benham, and Beck, 1885).
The Appendages
The segmental appendages of the scorpion include the chelicerae, the pedipalps and the legs of the prosoma, and the pectines of the abdomen. The prosomatic appendages will best be studied beginning with the legs, because the chelicerae and the pedipalps have fewer segments and their reduced segmentation can be more easily interpreted after a study is made of the legs and their musculature.
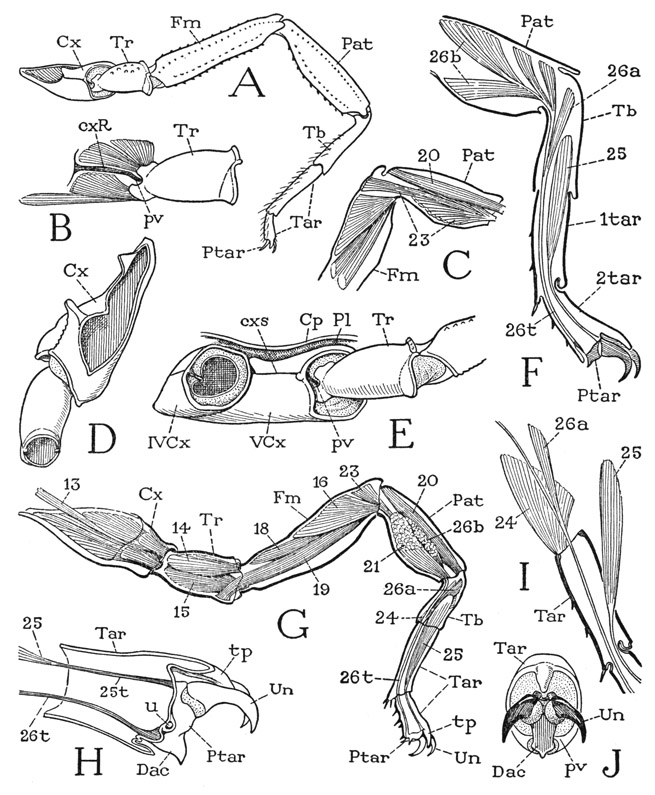
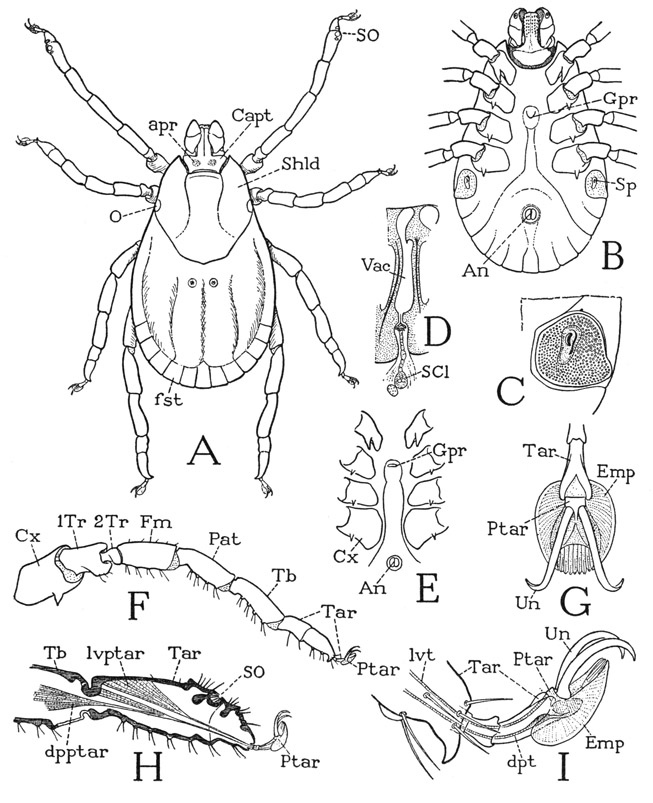
The Legs— The leg of the scorpion (fig. 19 A) is divided by flexible joints into eight podomeres (leg sections), but since the two parts in the tarsal region (Tar) are not connected by muscles, they are to be regarded as subsegments of the tarsus, or tarsomeres. The seven true leg segments, therefore, beginning at the base, are the coxa (Cx), trochanter (Tr), femur (Fm), patella (Pat), tibia (Tb), tarsus (Tar), and pretarsus (Ptar). The pretarsus is visible principally as a pair of claws, the body of the segment being mostly concealed in the end of the distal tarsomere (F, Ptar).
The coxae, as already noted, occupy most of the undersurface of the prosoma (fig. 17 B). Those of the first legs (1L) are short, but the others become successively longer and more oblique, the last pair being particularly long and slender. The coxae of the first and second legs have each a large mesal lobe, or endite (cxnd), projecting forward beneath the mouth. The coxae have no points of articulation on the body, and, except those of the first pair, are but little movable; yet, as shown by Beck (1885), they all have a strong musculature, including muscles from the carapace and from the endosternum, inserted on their bases or on basal apodemes. The long ventral surfaces of the second, third, and fourth coxae reach to the mid-line of the body, but the dorsal and lateral surfaces are relatively short (fig. 19 A, D, Cx). On its outer side the coxal wall is marked by a longitudinal groove (E, cxs) forming a strong internal ridge (B, cxR) that runs out posteriorly in a pivotlike process (B, E, pv), on which alone the trochanter is articulated. Because of the immobility of the coxae, the principal basal movement of the scorpion’s legs is at the coxotrochanteral joints.
Fig. 19. Arachnida—Scorpionida. The legs.
A, Centruroides sp., third left leg, anterior. B, same, trochanter and its muscles. C, same, patella and distal half of femur (muscles 21 and 26 removed, see G). D, coxa and trochanter of third left leg, dorsal. E, same, base of third leg and coxa of second leg, lateral. F, Pandinus sp., leg segments beyond femur, showing distribution of pretarsal muscles. G, same, third left leg, showing muscles visible in anterior view. H, same, pretarsus and its muscle tendons. I, Centruroides sp., base of tarsus, with single muscle (24) attached ventrally on posterior side of base. J, same, end view of tarsus and pretarsus.
For explanation of lettering see pages 126–127.
The trochanter is movable on the coxa by a great mass of muscle fibers inserted on the entire periphery of its base (fig. 19 G). The fibers, however, can be separated into at least 12 distinct muscles taking their origins in the coxa. The trochanter, therefore, is movable in any direction, but the lateral or forward movement is restricted by the position of the articular pivot and is dependent on the small muscles of the short anterior wall of the coxa (B) inserted above and below the articulation. The forward reach of the scorpion’s legs is due to the more or less horizontal position of the extended limbs, by which flexion at the joints becomes anterior instead of ventral. A slender muscle from the body (G, 13) that runs through the coxa is probably the “plastro-deutomeral” muscle of Beck (1885), but it tapers distally into a tendon that traverses the trochanter and is attached on the dorsal rim of the base of the femur.
The elongate femur is hinged to the trochanter by a strong, horizontal, dicondylic articulation that permits movement only in a dorsoventral direction. It is accordingly provided with a dorsal levator muscle (fig. 19 G, 14), and a two-branched ventral depressor muscle (15) arising in the trochanter, in addition to the slender levator (13) arising in the body, apparently on the endosternum.
The patella is an important segment of the scorpion’s leg; its interpolation between the femur and the tibia gives the leg its “double knee.” The femoropatellar articulation is a dicondylic hinge with a horizontal axis, but the active movement of the patella is downward on the end of the femur, since it has only depressor muscles. A wide, fan-shaped anterior depressor (fig. 19 G, 16) and a similar posterior depressor (17, not seen in the figure) arise in the distal part of the femur; a long ventral depressor (18) comes from the base of the femur, and another (19) takes its origin ventrally in the end of the trochanter.
The patellotibial joint again has a transverse hinge line between anterior and posterior articulations, and the short tibia turns abruptly downward from the end of the patella, but it has both flexor and extensor muscles. A single long extensor (fig. 19 G, 20) arises dorsally in the base of the patella; two lateral flexors (21, and 22 not seen in the figure) arise on opposite sides of the patella, while a ventral flexor (C, 23) has spreading fibers on the ventral wall of the patella and a median bundle arising in the distal end of the femur.
The long basal subsegment of the tarsus in the scorpion here described, Centruroides, has an anterior articulation on the tibia and is provided with but one muscle, a depressor (fig. 19 G, I, 24), which arises posteriorly in the tibia and is inserted ventroposteriorly on the base of the tarsus. No muscles are present between the two tarsomeres, though the latter are united by a flexible joint. From the end of the short distal tarsomere a fingerlike process (G, tp) projects over the pretarsus.
The claw-bearing pretarsus is often not recognized to be a true segment of the limb, but its structure as seen when fully exposed (fig. 19 H) leaves no doubt of its segmental nature. The body of the segment is a short but complete ring with anterior and posterior articular sockets that receive pivotal processes of the tarsus (J, pv), and it is provided with antagonistic dorsal and ventral muscles attached on its base by strong tendons (H, 25t, 26t). Ventrally the pretarsus is produced into a short median claw, or dactyl (Dac), which suggests that it corresponds with the terminal dactylopodite of a crustacean leg. The curved paired claws, which may be designated the ungues (Un), are set on membranous bases and are therefore flexible on the pretarsus, but they have no means of independent movement. The levator, or extensor, muscle of the pretarsus arises in the tibia (F, G, 25), the depressor, or flexor, has a branch in the tibia (26a), but the tendon continues into the patella and gives attachment to several fiber bundles (F, 26b) arising in the patella. The pretarsus thus has a rocking movement in a vertical plane on its tarsal articulations, which turns the claws up and down. The large size of the depressor muscle gives the claws the necessary strength on the downstroke. It seems remarkable that 26 muscles should be required to operate a single leg of the scorpion, in addition to those inserted on the coxa.
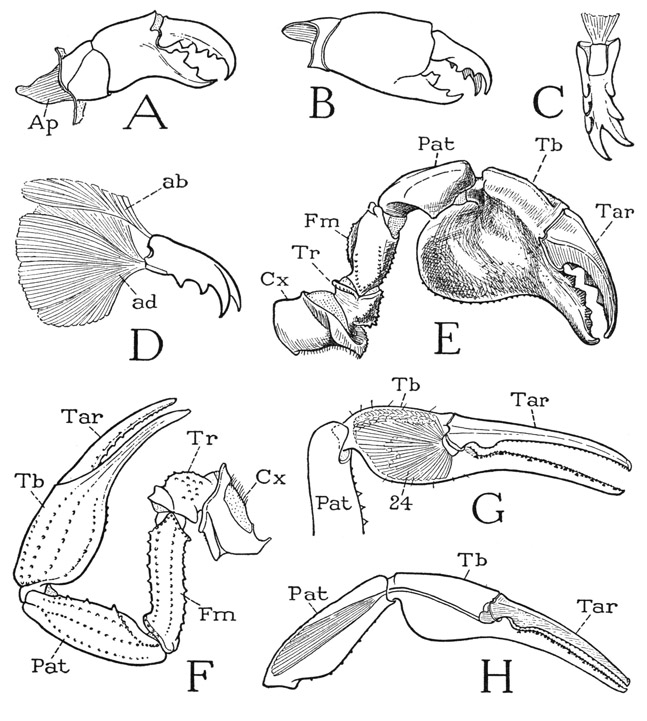
Fig. 20. Arachnida—Scorpionida. The chelicerae and the pedipalps.
A, Pandinus sp., chelicera. B, Centruroides sp., chelicera. C, same, movable finger of chelicera, ventral. D, same, movable finger of chelicera and its muscles. E, Pandinus sp., right pedipalp, ventral. F, Centruroides sp., left pedipalp, dorsal. G, same, chela of pedipalp, showing tibial muscles of movable finger (Tar). H, same, showing patellar muscle of movable finger.
For explanation of lettering see pages 126–127.
The Pedipalps— The scorpion’s pedipalp has only six segments, including the movable finger of the chela (fig. 20 E, F), and thus lacks a segment present in the legs. It is therefore of interest to determine what segment is missing. By comparison with a leg the first three segments of the pedipalp must be the coxa (Cx), the trochanter (Tr), and the femur (Fm). If we next examine the musculature of the movable finger within the “hand” of the chela (G), we find that the latter is filled with a great mass of fibers all attached on a ventral process of the base of the movable finger. If the finger represents the pretarsus of a leg, it should have an opening as well as a closing muscle. The segment of the leg that has a single muscle, and that one a flexor, is the tarsus (fig. 19 I, 24). We must conclude, therefore, that the “hand” of the pedipalp chela is the tibia (fig. 20 E, F, G, Tb), the movable finger the tarsus (Tar), and the segment that supports the chela the patella (Pat). The movable finger, of course, may be supposed to include the pretarsal claw in its tip, there being evidence of this in some other arachnids with chelate pedipalps. The strength of the movable finger is reinforced by another muscle arising in the patella (H) and inserted by a strong tendon on the basal knob of the finger. It is surprising perhaps that the chela should not have an opening muscle, but evidently the elasticity of the hinge of the finger on the hand keeps the forceps open until closed by the finger muscles. In contrast to the scorpion chela, the similar chela of the crayfish (fig. 45 B) has both an opening and a closing muscle, and the movable finger is the pretarsus, or dactylopodite.
The Chelicerae— The chelicerae of the scorpion (fig. 20 A, B) are relatively short but powerful pincers. They are three-segmented, and since the chelicerae of none of the Chelicerata have more than three segments, there is no apparent way of identifying the segments. The strongly toothed movable finger of the cheliceral appendage has both an opening and a closing muscle arising in the hand (D), and hence is comparable to the finger of a crustacean chela, which is the dactylopodite. The structural uniformity of the chelicerae in all the chelicerate arthropods would indicate that these appendages have been handed down in their present form from some remote ancestor of the group.
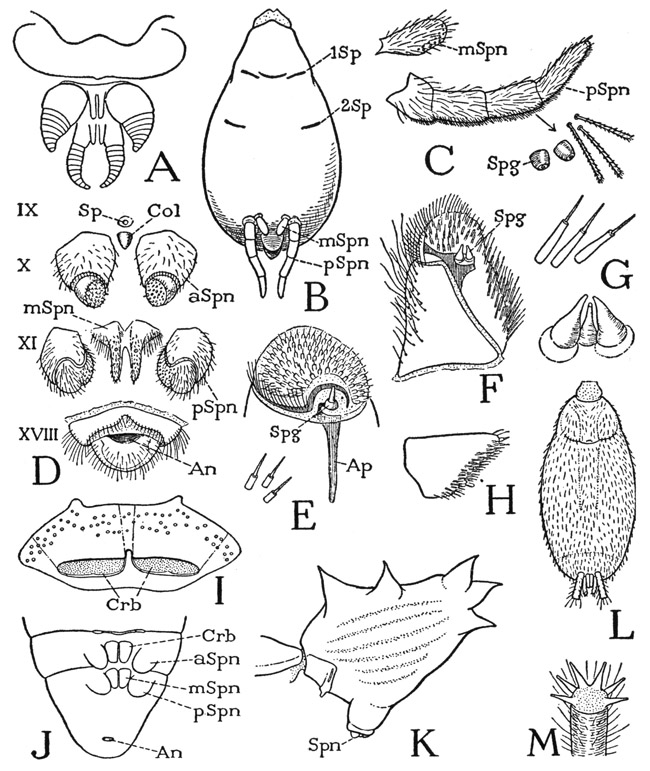
The Pectines— The comblike organs borne on the base of the undersurface of the abdomen (fig. 17 B, Pec), known as the pectines, are movably attached by their bases to the sternal plate of the ninth segment (B, E, IXS), and are regarded as the appendages of this segment. They vary in form in different genera of scorpions, but a typical example of their structure is that shown at E in Pandinus. Each pecten bears an anterior row of long teeth, and a posterior row of small teeth. The pectines are peculiar to the scorpions; their specific function is not known, but they are unquestionably important sensory organs, since the teeth bear numerous innervated sensilla. Immediately in front of them is the genital opening, or gonotreme (E, Gtr).
The Feeding Apparatus, Digestion, and Excretion
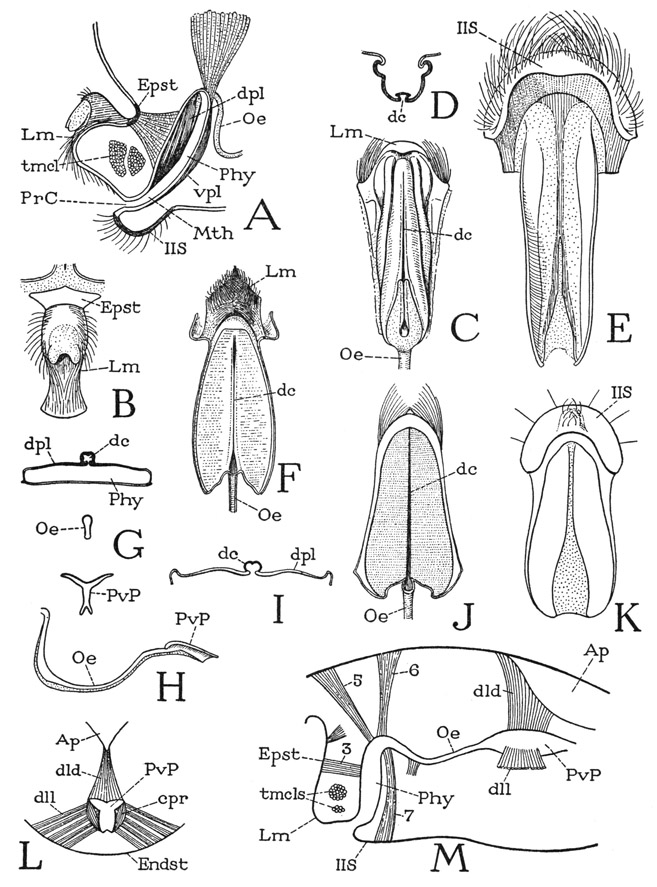
The mouth of the scorpion is concealed within a large, open preoral cavity between the broad, soft inner surfaces of the pedipalp coxae (fig. 21 A, PrC). The cavity is overhung dorsally by the chelicerae (Chl), but ventrally it is closed by a wide underlip formed of the closely approximated endites of the coxae of the first and second legs (1cxnd, 2cxnd). When the chelicerae and the pedipalps are removed (B), the mouth is seen to be a small aperture (Mth) beneath the base of the large, laterally compressed labrum (Lm) that projects forward from between the bases of the pedipalps. Extended anteriorly from below the mouth is the basinlike underlip, the upper part of which is formed of the concave dorsal surfaces of the first coxal endites (B, C, cxnd). The opposing edges of these two endites are not in contact but leave between them a median groove, which is closed below by the long, rigid supporting endites of the second leg coxae (A, 2cxnd). The gutterlike groove leads directly into the mouth. The relation of these preoral structures to one another and to the mouth is seen in the longitudinal section shown at D. The labrum (Lm) has a rounded dorsal surface terminating in a fringe of long hairs, below which the anterior wall slopes back to the short ventral surface that overhangs the mouth (Mth). Crossing the inner part of the cavity of the labrum are two bundles of transverse compressor muscle fibers (tmcl). At the base of the labrum, dorsally, is the thick, irregular epistomal sclerotization of the head integument (Epst); on each side it gives off into the body cavity a long epistomal apodeme (eAp, only the one on the right seen in the figure). Beneath the labrum and the mouth is the floor of the preoral cavity composed of the endites of the first and second leg coxae.
The mouth leads into a small pear-shaped pouch (fig. 21 D, Phy) that enlarges upward from its narrowed entrance at the mouth. This pouch is the sucking organ known as the pharynx in arachnology. It is somewhat compressed laterally and rounded at its inner end; the slender oesophagus (Oe) departs from its lower wall at the end of a ventral channel from the mouth. The dorsal wall is deeply infolded lengthwise, and the trough of the invagination is strengthened by an elastic rod. Compressor muscles cover the walls of the pharynx; dilator muscles (dld) attached on the concave dorsal wall arise on the epistomal sclerotization (Epst) at the base of the labrum, and lateral dilators have their origins on the epistomal apo-demes (eAp).
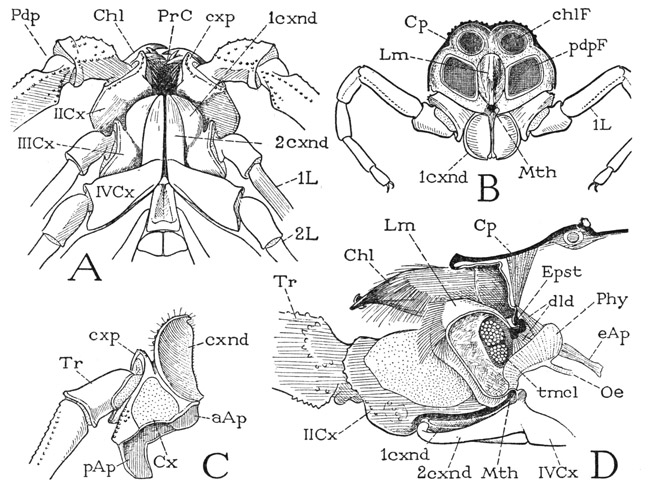
Fig. 21. Arachnida—Scorpionida. Mouth parts of Centruroides sp.
A, ventral side of anterior part of body, showing mouth parts surrounding a large preoral food cavity (PrC). B, anterior end of body, chelicerae and pedipalps removed, exposing the mouth (Mth) and coxal endites of first legs. C, base of first left leg with coxal endite, dorsal. D, longitudinal section through anterior end of body and labrum, right chelicera and base of right pedipalp in place, showing mouth leading into pharynx.
For explanation of lettering see pages 126–127.
The preoral cavity of the scorpion, being open dorsally directly under the short chelicerae, serves as a receptacle for liquids or fragments of the prey held and crushed in the cheliceral pincers. There is no straining apparatus guarding the mouth, such as is present in many other arachnids, but the small size of the scorpion’s mouth precludes the entrance of large pieces of food, and the scorpion, in common with all other arachnids, has only a sucking apparatus for the ingestion of food.
In each of the orders of Arachnida the mouth parts are different, as shown elsewhere by the writer (1948), but in all except the Palpigradi they are simply modifications of the same structures that compose the feeding organs of the scorpion. On the other hand, there is a radical difference between the arachnids and Limulus in the structure of the oral region of the body, the ingestion apparatus, and the manner of feeding. The arachnids feed on liquids extracted from the prey either mechanically or by extraoral digestion, and their organ of ingestion is a sucking pump; Limulus devours pieces of animal food, which are ground up in a proventricular gizzard.
Scorpions in captivity will eat any kind of small arthropod. The prey is seized by the chelae of the pedipalps, which in large species are able to crush hard-shelled beetles, but if the victim is not killed by crushing, it is subdued by the abdominal sting. From the pedipalps the food is passed to the chelicerae, one of which holds it while the other rips open the body and pulls out the viscera. The extracted material collected in the preoral cavity is thoroughly cut up by the chelicerae, then reduced to a pulp by digestive juices discharged upon it, probably from the stomach, and finally in liquid form it is sucked into the mouth by the pharynx. A detailed account of the feeding of a scorpion is given by Kästner (1940, pp. 154–158).
The stomodaeal oesophagus leads into the mesenteron, which consists of a tubular stomach section lying in the prosoma and mesosoma, and of a long intestinal section that begins in segment XII and extends into the last tail segment, where it joins the very short proctodaeum that opens through the anus. From the sides of the axial tube of the stomach are given off six pairs of diverticula that expand into large sacs with soft, infolded walls, all of which are closely packed along the sides of the body and bound together by a covering tunic of connective tissue. The first pair of diverticula is in the prosoma, the others arise in the first five segments of the mesosoma, but those of the last pair are branched and extend into the base of the metasoma. These diverticular sacs of the stomach form the major part of the alimentary system of the scorpion and occupy most of the space in the mesosoma. The digestive processes of the scorpion have been described by Schlottke (1934). The epithelial walls of the stomach sacs include secretory cells and digestive cells. The first produce enzymes that are given off into the lumina of the sacs and accomplish a preliminary digestion of the food pulp received from the pharynx; according to Pavlovsky and Zarin (1926), the digestive enzymes of the scorpion include amylase, lipase, and proteinases. The digestive cells absorb the products of enzyme action, and within them takes place the final stage of digestion. The cells at last become filled with excretory granules, which are thrown out and discharged through the intestine.
Connected with the mesenteron are two pairs of excretory tubes, known as Malpighian vessels, which remove waste matter from the blood; one pair branches in the mesosoma, the other goes into the prosoma. In addition, the scorpion has a single pair of coelomic excretory glands lying in the posterior part of the prosoma, the ducts of which open in the grooves between the coxae of the third legs and the prosomatic sternal plate.
The Respiratory Organs
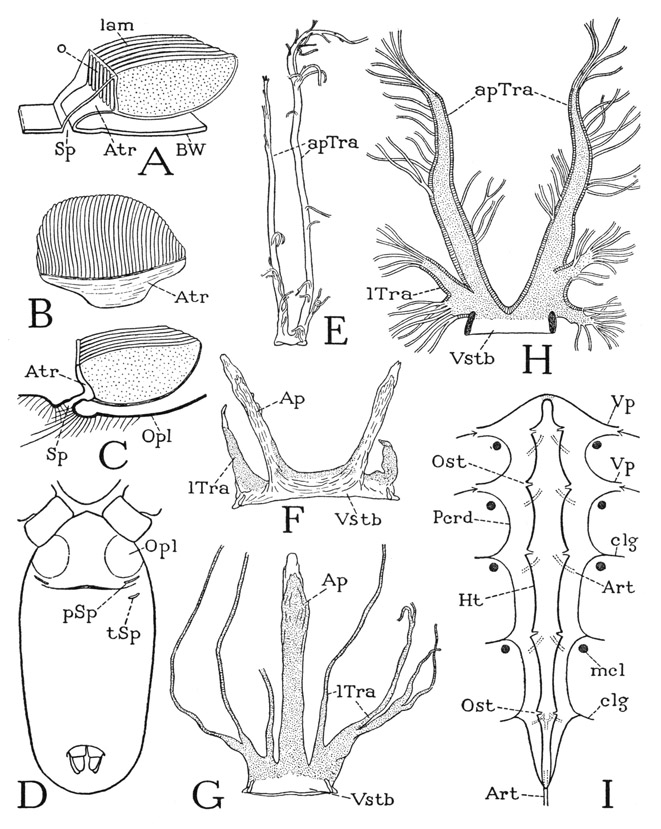
The breathing apparatus of the scorpion consists of four pairs of respiratory organs located inside the abdomen above the sterna of segments X to XIII (fig. 22 B, bl) and opening by slitlike apertures, the spiracles, on the lateral parts of the sterna of these same segments (C, Sp). The organs are known as book lungs because the essential parts of them consist of numerous thin, hollow, leaflike lamellae attached on a common base like the leaves of a book (D). The spiracle of each organ (E, Sp) opens into an obliquely elongate atrial chamber (D, E, Atr), which is produced beyond each end of the spiracle in a tapering extension. The anterior and posterior walls of the atrium are membranous and flexible, but the arched dorsal wall is crossed by closely set, longitudinal bars (F, s), which are the septa between narrow slits (o) opening into the lumina of the lamellae (lam). The lamellae are somewhat triangular in shape (E), set vertically on the atrium, and extend anteriorly from it. The atrium and the leaflets are ingrowths of the body wall, and are lined with a delicate cuticle which is drawn out and renewed at each moult. In the species illustrated the leaflets appear to be entirely free from one another and can be readily spread apart (F), but in some scorpions they are said to be united by protoplasmic strands of their epidermal walls.
Each lung is enclosed in a pulmonary cavity, or sinus, of the haemocoele covered by a sheet of connective tissue. The lumina of the leaflets contain the respiratory air derived from the atrium, and the blood circulates in the spaces between the lamellae, the gas exchange taking place through the very thin walls of the latter. Air enters the leaflets probably by diffusion from the atrium, but the atrium is said, in some species at least, to have a ventilating action by means of muscles. According to Fraenkel (1929), in a species of Buthus there are two muscles attached on the posterior wall that produce an opening of the spiracle and an expansion of the atrium, the closing being automatic on relaxation of the muscles. The opening of the spiracles, Fraenkel observes, takes place only when the scorpion is active. The wall of each pulmonary sinus is connected with the pericardium in the dorsal part of the abdomen by a strand called the “pericardio-pulmonary muscle” by Lankester (1885), but which Fraenkel says is not muscle tissue; it transmits to the sinus, however, the vibrations of the heart beat, which cause a rapid pulsation of the lung in the sinus, and probably thus aids the circulation of blood between the lung lamellae.
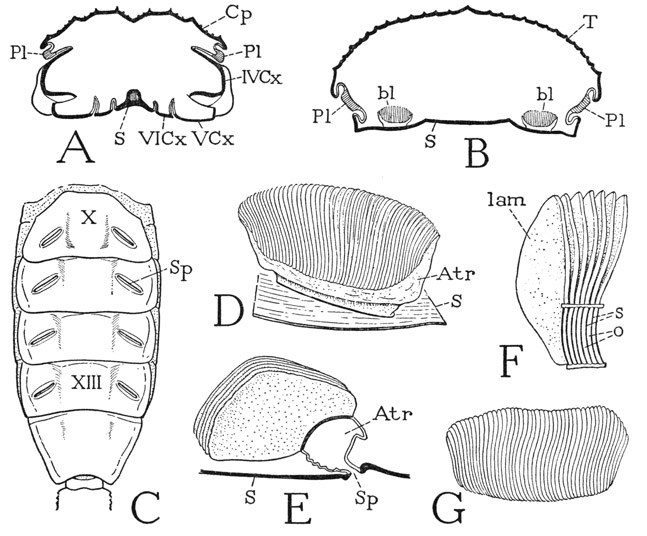
Fig. 22. Arachnida—Scorpionida.
A, Centruroides sp., outline of cross section of prosoma through coxae of second legs (IVCx), showing pleural folds (Pl) between carapace and coxae. B, same, outline of cross section through a mesosomatic segment, showing position of book lungs. C, Pandinus sp., ventral surface of mesosoma. D, same, right book lung of segment X, dorsoposterior view. E, same, vertical section of book lung, showing spiracular entrance to atrium. F, same, part of anterodorsal wall of atrium, showing slitlike openings (o) into lung lamellae. G, same, book lung, dorsal.
For explanation of lettering see pages 126–127.
The respiratory organs of Arachnida in general include book lungs and tubular tracheae. The scorpion has the greatest number of lungs; some other arachnids have not more than two pairs and, where only one pair is present, the lungs are supplemented by tracheae; but some arachnids have tracheae only. Those zoologists who formerly contended that the arachnids are derived from merostomes attempted to explain the arachnid lungs as invaginated gills such as those of Limulus. The theory, however, would have to assume that the individual lamellae of the gill have been turned outside-in to form the leaflets of the lung, an assumption that in itself is enough to discredit the theory, and, moreover, the gill leaflets of Limulus are transverse, while the lamellae of the arachnid lung are longitudinal and vertical.
A comparison of one of the tailed eurypterids (fig. 13 D, E) with a scorpion shows a rather striking superficial resemblance between the two, which is accentuated when the comparison is with a Silurian fossil scorpion (fig. 17 D), since the short legs of the latter much resemble those of the eurypterid. A close relationship between scorpions and eurypterids, therefore, has been almost taken for granted, but with a difference of opinion as to which is the ancestral form. The scorpion, however, is by no means a primitive arachnid, as Versluys and Demoll (1920) have emphatically stated; its feeding organs are specialized in the arachnid manner, and very probably those of the eurypterids were quite different, certainly the method of feeding and the structure of the mouth parts of Limulus are not arachnoid. Considering these points and others of equal importance, such as the radical differences between external gills and internal lungs, the idea of a close relationship either way between the merostomes and the arachnids is difficult to maintain. The two groups may be regarded as members of the subphylum Chelicerata, but their ancestry cannot be traced to any known common progenitor.
Comparison of a Scorpion with the Palpigradi
The most primitive arachnid structure known is to be seen in the members of the Palpigradi, a group of minute spiderlike forms including the genus Koenenia (fig. 23 A) found in the Mediterranean region of Europe, Prokoenia from Texas and California, and a fossil Jurassic species named Sternarthron (C).
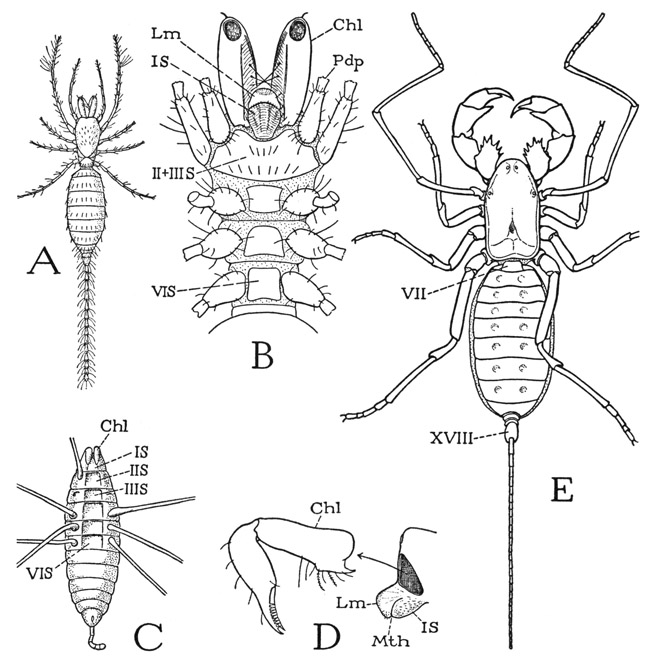
Fig. 23. Arachnida—Palpigradi (Koenenia) and Pedipalpida (Mastigoproctus).
A, Koenenia mirabilis Grassi (from Plansen and Sörenson, 1897). B, same, prosoma and bases of appendages, ventral (from Börner, 1901). C, Sternarthron zitteli Haase (var. minus Oppenheim), a Jurassic fossil palpigrade (from Haase, 1890). D, Koenenia mirabilis Grassi, anterior end of body with mouth cone and detached left chelicera (from Hansen and Sörensen, 1897). E, Mastigoproctus giganteus (H. Lucas).
For explanation of lettering see pages 126–127.
A palpigrade at first sight (fig. 23 A) has a superficial resemblance to a scorpion in the presence of a long jointed tail; but a closer inspection shows that the rings of the tail are not body segments, and that the tail itself is an appendage of the last segment of the body proper, being similar to the caudal flagellum of the whip scorpion (E), which is borne on a short three-segment stalk comparable to the postabdomen of the scorpion. It is in the anterior part of its body that the palpigrade shows its primitive features. The appendages corresponding with the huge chelate pedipalps of the scorpion are slender legs (A) arising entirely behind the mouth (B, Pdp) from a large sternal plate (II + IIIS) that carries the next long leglike appendages and is followed by three separate sternal plates of segments IV, V, and VI. The “pedipalps” of the palpigrades thus form no part of the feeding apparatus; in all other arachnids their coxae are intimately associated with the mouth. The mouth of the palpigrade is situated on a small, snoutlike projection of the head end of the body (D, Mth), and the chelicerae arise just above the base of the snout.
The dorsal surface of the snout of Koenenia is evidently the labrum (Lm), the ventral surface would appear to be, as Börner (1901) has interpreted it, the sternum of the cheliceral segment (IS). The organ contains the sucking pharynx. In the fossil Sternarthron (C), as illustrated by Haase (1890), there are shown six distinct sternal plates on the venter of the prosoma. In no other arachnid order is a cheliceral sternum present, or recognizable as such in the adult, though it is present in the embryo. There is no question with arachnologists that the Palpigradi present the most primitive structure of the prosoma known among the arachnids. It follows, then, that the scorpion is entirely too specialized to qualify as a modern representative of the arachnid ancestors.
SPIDERS
The arachnids commonly known as spiders belong to the order Araneida, or Araneae. In some respects the spiders are the most remarkable product of the arthropod phylum. In their instincts they equal or surpass the insects, and as spinners and weavers of silk they have no rivals. The cocoon of a silkworm and the unsightly domiciles of the webworms and tent caterpillars are but crude things compared with the geometric webs of the orb-weaving spiders. “The orb web,” says Gertsch (1949) “would seem to stand alone as a glorious creation, an incredible novelty designed by superior artisans.” Yet, there is no evidence that the intricate activities of the spiders in the construction of their silken snares and webs for catching prey and in their extraordinary modes of mating are guided by any faculty other than that of “blind instinct”; two spiders of the same species are never known to do the same thing in different ways.
Anatomically the spiders are equally remarkable for the structural adaptations that subserve their instincts. In no other arachnid is the body so narrowly constricted between the leg-bearing prosoma and the abdomen, which carries the spinning organs. No other arachnid has abdominal silk-producing glands; the spinnerets have been evolved from a pair of segmental appendages, with accessory structures between them. In the male spider the apical segment, or the last two segments, of the pedipalps have been elaborated, often to an extreme degree, into a complex structure for transferring sperm to the female, who, in turn, is provided with receptacles that in intricacy match the intromittent organs of the male. On the other hand, in most other respects, the spiders are simply arachnids: their feeding aparatus is in no way specialized; the alimentary canal and the respiratory, circulatory, and reproductive organs are essentially those of the arachnids in general.
General External Structure
In a typical spider the prosoma is relatively small and depressed as compared with the rotund abdomen, which is attached to the prosoma by such a narrow pedicel that it is freely movable in all directions. The pedicel is traversed by the alimentary canal, the aorta, tracheae, and nerve trunks. The nerve centers of the spiders all lie in the prosoma, where the ganglia of the nerve cord are condensed in a large suboesophageal nerve mass closely united with the brain around the sides of the oesophagus. The abdomen contains the heart, most of the stomach and its diverticula, the intestine, the Malpighian tubules, the respiratory organs, the silk glands, and the reproductive organs.
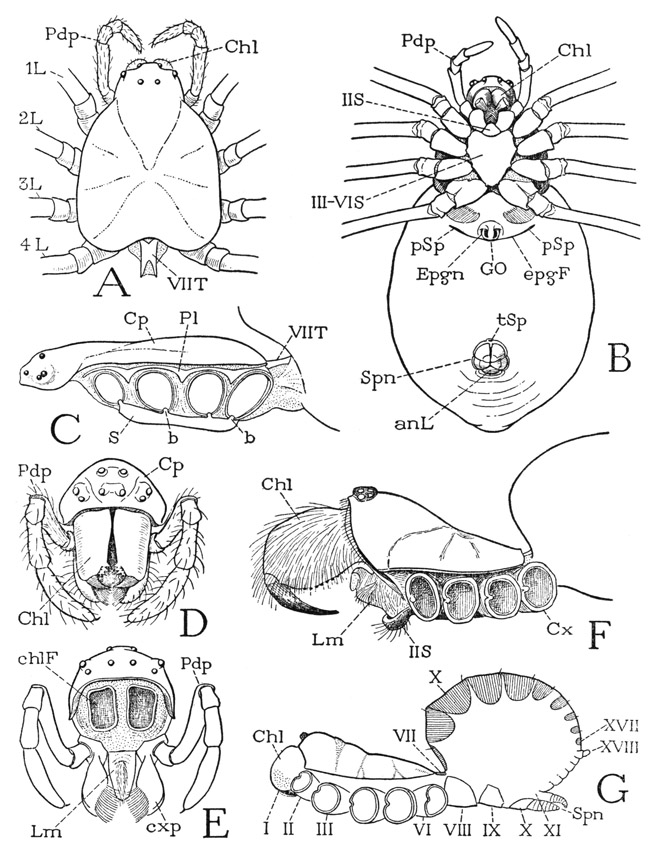
The prosoma (fig. 24 A), as in other arachnids, carries the chelicerae (Chl), the pedipalps (Pdp), and the four pairs of legs (L). Its dorsal surface is covered by a leathery carapace, on which indistinct lines radiating from the center suggest a division into a “head,” bearing the eyes, chelicerae, and pedipalps, and four segments corresponding to the legs. The V-shaped anterior line is called the “cervical groove,” and the part behind it the “thorax,” but there is nothing in the development of any arachnid to indicate that the spider was ever divided in this manner into a head and a thorax. The embryonic head of an arachnid lies in front of the cheliceral segment, and the eyes pertain to it, but all the prosomatic appendages belong to postcephalic body segments. However, inasmuch as these segments in the adult are intimately united with the embryonic head, the prosoma of the spider may appropriately be termed a cephalothorax. Most spiders have eight eyes distributed across the anterior part of the carapace (D), but in some all the eyes are grouped on a median tubercle (F, G). The number of eyes, however, may be reduced to six, four, or two, and some cave-inhabiting spiders have no eyes. The number, position, and relative size of the eyes serve as diagnostic characters for classification.
The body wall in front of the carapace abruptly descends as a wide membranous area from which the chelicerae arise (fig. 24 E), and ends below between the bases of the pedipalps, where it supports the pendent labrum (Lm). In most other arachnids the labrum is attached on a sclerotic bridge, the epistome, uniting the pedipalp coxae. A small epistomal plate is present in some of the mygalomorph spiders (fig. 31 B, Epst), but in most species the epistomal region above the labrum is unsclerotized or not distinctly separated from the labrum (fig. 27 F, G). Araneologists commonly call the labrum the “rostrum,” and give the name “labrum” to the anterior part of the carapace before the eyes, but this usage of the term labrum is clearly a misapplication, since the labrum of all arthropods is a free lobe overhanging the mouth, though in ordinary spiders it is concealed behind the chelicerae, which hang downward from beneath the front of the carapace (fig. 24 D). The lumen of the labrum is crossed by two compressor muscles (fig. 31 A, M, tmcls); within it also is a gland or pair of glands. According to Petrunkevitch (1933), there are probably two labral glands, or so-called “rostral” glands, present in all spiders, but in some they are so closely united as to appear to be a single organ; the two ducts discharge into a wide, slitlike atrium that opens on the anterior surface of the labrum. The structure of the labral glands in Atypus piceus Sultzer is described in detail by Bertkau (1885).
Fig. 24. Arachnida—Araneida. General Structure.
A, Argiope trifasciata Forsk., female, prosoma and pedicel, dorsal. B, same, entire body, ventral. C, same, prosoma with appendages detached, lateral. D, same, anterior end of body, showing eyes, chelicerae, and pedipalps. E, same as D with chelicerae removed. F, Eurypelma hentzi Chamb., female, prosoma and base of opisthosoma, pedipalps removed, legs cut off beyond coxae, lateral. G, Liphistius desultor Schiödte, female, showing complete arachnid body segmentation (from Bristowe and Millot, 1932).
For explanation of lettering see pages 126–127.
The undersurface of the prosoma of most spiders, as seen in Argiope (fig. 24 B), is formed of a small sternal plate between the pedipalp coxae and of a large, usually heart-shaped plate between the leg coxae. The small anterior plate (IIS) is the sternum of the pedipalp segment; the second plate bears on each margin (C) four small knobs on which the leg coxae are articulated, and therefore represents the combined sterna of the four leg-bearing segments (B, III–VIS). Since the pedipalp sternum lies immediately below the mouth (fig. 31 A, Mth) and serves the spider as an underlip, it is commonly called the “labium,” though it has no homology with the labium of an insect, which is formed of the appendages corresponding with the second pair of legs of an arachnid. In some spiders the pedipalp sternum is united with the leg sternum (fig. 27 I). The legs of the spider arise from the membranous lateral walls of the prosoma between the carapace and the sternum (fig. 24 C, F). Intervening between the carapace and the coxae there may be a narrow sclerotic band (C, Pl), which, though it does not give articular supports to the coxae, may be regarded as a pleural sclerotization.
The araneid opisthosoma, or abdomen, varies in shape from globular to elongate, or takes on irregular and sometimes bizarre forms. In the Liphistiomorpha the abdomen is shown definitely, by the presence of distinct tergal plates (fig. 24 G), to be composed of 12 segments, including the anal lobe (XVIII), but in the other spiders the abdominal segmentation is obscured or obliterated in the adult, though certain external features are always associated with specific segments. The first opisthosomatic segment is the abdominal pedicel (A, C, VIIT). The dorsum of the abdomen has no special characters; on the venter (B) are located the genital opening, the apertures of the respiratory organs, the spinnerets, and, in the female, the orifices of the sperm receptacles.
Crossing the anterior part of the ventral surface of the abdomen is a groove known as the epigastric furrow (fig. 24 B, epgF). In the middle of the furrow is the simple genital opening (GO) in both sexes, and in the lateral parts, in the majority of spiders, the slitlike apertures (pSp) of a pair of book lungs. Before the genital opening of the female of most spiders is a strongly sclerotized plate termed the epigynum (Epgn), which contains the openings of the sperm receptacles. In the four-lunged Liphistiomorphae, Mygalomorphae, and Hypochilomorphae, a second pair of pulmonary spiracles lies a short distance behind the first pair (fig. 34 B, 2Sp), but in the two-lunged spiders the second lungs are replaced by tracheae. The tracheal spiracles may lie in the position of the second lung spiracles, or more centrally on the abdominal venter, but usually they come together in a common posterior opening just before the spinnerets (fig. 24 B, tSp). In a few spiders the first lungs also are replaced by tracheae. The first pair of respiratory organs pertains to the second abdominal segment (VIII), the second pair, whether lungs or tracheae, belongs to segment IX, which on the venter extends back to the spinnerets.
The spinnerets (fig. 24 B, Spn) are a group of small appendages, usually six of them, in most spiders lying close before the anal lobe (anL). The spinnerets pertain to segments X and XI, but the anal lobe represents segment XVIII; the ventral arcs of the six intervening segments, therefore, are compressed in the narrow space between the spinnerets and the anal lobe, except in Liphistius (G), in which there is a long segmented area of the abdominal venter behind the spinnerets. The abdomen of most spiders projects more or less beyond the spinnerets, but the surface seen from below behind the anal lobe (B) is a part of the dorsum.
The respiratory organs, the epigynum, and the spinnerets will be more fully described in following sections.
The Legs
The legs of spiders are so attached on the sides of the prosoma between the carapace and the sternum that they turn anteriorly and posteriorly. There may be no specific articulation of the leg base on the body, but usually the coxae are articulated ventrally on marginal knobs of the sternum (fig. 24 C, b). The extrinsic leg muscles include dorsal muscles arising on the carapace, and ventral muscles from the endosternum.
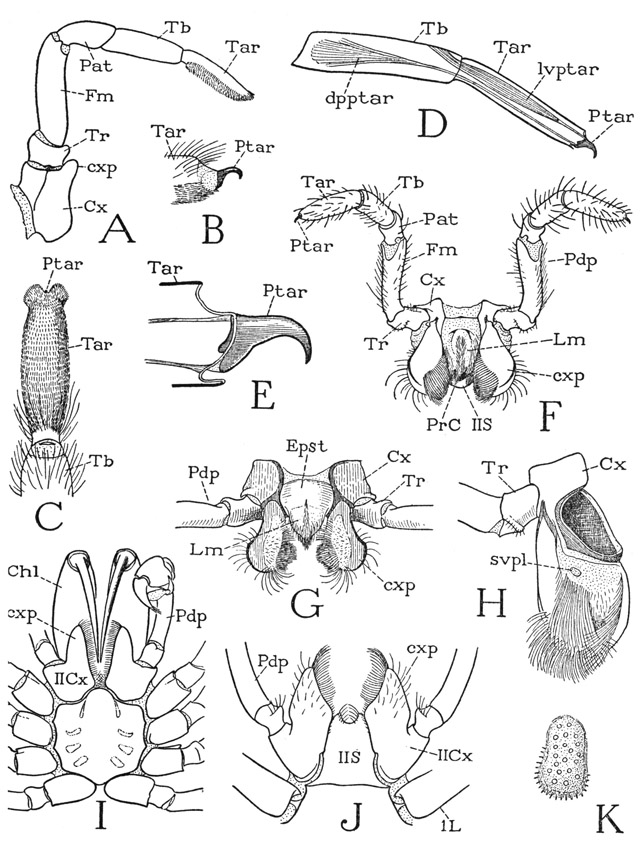
The spider leg (fig. 25 A) consists of seven true segments, but the small terminal segment, or pretarsus (Ptar), bearing the claws is mostly concealed by hairs, or by retraction into the end of the tarsus. The other segments, beginning at the base of the leg, are the coxa (Cx), a single trochanter (Tr), the femur (Fm), a short patella (Pat), the tibia (Tb), and the tarsus (Tar). The tarsus, however, is distinctly subdivided into a long basal part (1tar) and a shorter distal part (2tar). Arachnologists commonly term the basal tarsomere the “metatarsus” and the distal tarsomere the “tarsus,” though by analogy with vertebrate anatomy the two names should be reversed, and in entomology “metatarsus” would refer to the tarsus of a metathoracic leg. To avoid confusion, therefore, it will be better to call the basal tarsomere of the spider leg the basitarsus (1tar) and the distal one the telotarsus (2tar). That the two tarsal parts are not true segments is shown by the consistent absence of interconnecting muscles.
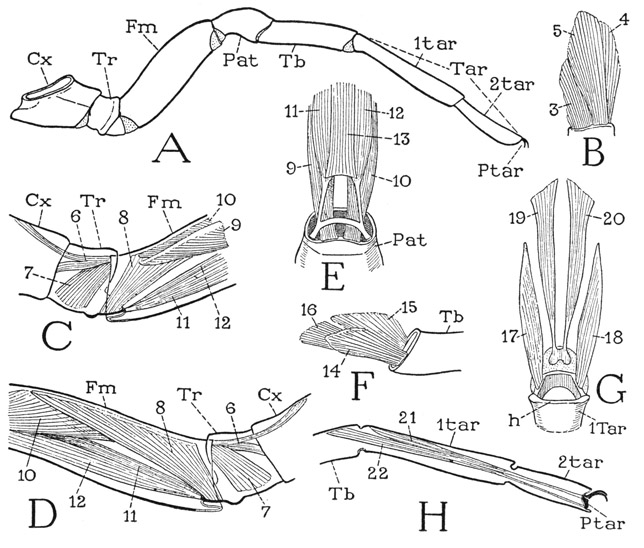
Fig. 25. Arachnida—Araneida. Segmentation and musculature of the legs of Eurypelma hentzi Chamb.
A, second left leg, anterior. B, ventral muscles of trochanter arising in coxa. C, muscles of femur (6, 7, 8) arising in trochanter and coxa, and proximal ends of patellar muscles (9, 10, 11, 12), anterior. D, same part of leg, posterior. E, base of patella and its muscles, ventral. F, base of tibia and its muscles from patella, anterior. G, base of tarsus and its muscles, dorsal. H, distal part of leg, showing subsegments of tarsus and distribution of pretarsal muscles.
For explanation of lettering see pages 126–127.
Differences in the articulations at the joints between the leg segments give to the leg a variety of movements. The trochanter, and therefore the telopodite as a whole, turn up and down on the end of the coxa; between these two segments there is a strong anterior articulation, but no specific posterior articular point. The trochanterofemoral joint is dicondylic with both anterior and posterior articulations, so that the femur moves on the trochanter in a vertical plane, though its principal flexion is in a dorsal direction. The patella is joined to the femur by a strong, transverse, dicondylic dorsal hinge; the femoropatellar joint is the “knee” of the spider leg, with a principal ventral flexion. The patellotibial joint differs from the other joints in that its axis is obliquely vertical with a dorsal point of articulation between the adjoining segments. Movement at this joint, therefore, is transverse to the axis of the limb, and the nature of the joint enables the patella in its up and down movement on the femur to carry the distal part of the leg with it. The basitarsus again moves in a vertical plane since it has a strong transverse dorsal hinge (fig. 25 G, h) on the end of the tibia. The distal tarsomere is freely flexible on the basitarsus, but there are no controlling points of articulation and no muscles at this intratarsal joint. At the end of the tarsus are the apical claws of the leg, commonly termed the “tarsal claws.” An examination of the foot of the spider (fig. 26), however, shows that, as in other arthropods, the claws pertain to a small end segment of the limb, which is the pretarsus, or dactylopodite, having its own muscles. The body of the pretarsus is set vertically in the articular membrane at the end of the tarsus, and in most spiders is produced in a median claw, or dactyl (C, D, E, Dac); the paired claws, or ungues (C, Un), are flexibly attached to the upper end of the pretarsus (Ptar).
The intrinsic musculature of the leg appears to be essentially the same in all spiders. In a recent study of the leg muscles of the tarantula Eurypelma, Dillon (1952) finds 31 muscles in all for each leg, 11 of which are in the coxa. This account is much more complete and accurate than any other hitherto published on the arachnid leg muscles. It is often difficult, however, to decide how many individual muscles may be represented in a compact mass of fibers having a common insertion. The writer, for example, has enumerated only five major groups of fibers in the coxa of Eurypelma, two being dorsal and three ventral (fig. 25 B). In the following descriptions of the muscles of the telopodite 17 muscles have been recognized instead of 20 as given by Dillon, but the 17 muscles shown on figure 25 will sufficiently illustrate the mechanism of the arachnid leg.
The coxal muscles operate directly the trochanter, but they serve as levators and depressors of the telopodite as a whole. The femur is individually movable in a vertical plane on the trochanter. Its levator muscles, attached dorsally on the base, include two groups of fibers, one a horizontal dorsal muscle (fig. 25 C, D, 6) with a short branch arising in the base of the trochanter, and a longer branch from the coxa, the other (7) is a thick bundle of obliquely dorsoventral fibers from the ventral wall of the trochanter. The depressor of the femur is a large muscle (8), the fibers of which arise on the extended lower lip of the trochanter and spread distally in the posterior part of the femur (D) to be attached dorsally in the proximal two-thirds of the segment. This muscle is present in dipneumone spiders examined, though more weakly developed than in Eurypelma. The patella has only depressor muscles (E). Two of them are large anterior and posterior muscles (9, 10) arising dorsally in the proximal part of the femur (C) and inserted directly on the lower lip of the base of the patella (E). Traversing the femur ventrally is a compact bundle of fibers attached anteriorly on the lower lip of the trochanter, which is separable into two thick lateral muscles (C, D, E, 11, 12) and a thin, flat ventral muscle (E, 13). Posteriorly the lateral muscles are attached separately by a pair of tendons to an arcuate bar in the ventral articular membrane at the base of the patella (E); the ventral muscle (13) is attached on the membrane itself. The lateral muscles of this group evidently are depressors of the patella; the median ventral muscle possibly pulls on the infolding membrane. The “knee” joint of the limb between the femur and the patella is an important point of ventral flexure.
The patella is fully occupied by three short muscles of the tibia (fig. 25 F), one anterior (14), one posterior (15), and one ventral (16), but the last has an anteroventral insertion; 14 and 16, therefore, are productors, 15 a reductor, the movements of the tibia on the patella being transverse to the axis of the leg. The basitarsus has four muscles (G), all effective as depressors because they are attached below the dorsal tibiotarsal hinge (h). An anterior and a posterior muscle (17, 18) are inserted on the base of the tarsus; a pair of ventral muscles (19, 20) is attached by tendons on a small plate in the ventral articular membrane of the joint. Between the two parts of the tarsus (H, 1tar, 2tar) there are no muscles, but the tarsus as a whole is traversed by the muscles of the pretarsus (Ptar), a dorsal muscle (21) arising proximally in the basitarsus, and a ventral muscle (22) arising dorsally in the distal end of the tibia. The mechanism of the pretarsus will be discussed in a following paragraph.
A study of the musculature of the spider’s leg, as has been noted by other writers, shows that there are no levator (extensor) muscles of the patella, the tibia, or the tarsus; the tibia has no upward movement by reason of the nature of its connection with the patella. Ellis (1944) has given reasons for believing that extension of the leg at the femoropatellar and tibiotarsal joints is produced by blood pressure, there being no evidence of elasticity at the joints, since the legs of a dead spider are always flexed. “Experimental evidence,” he says, “demonstrates that extension of the leg is intimately associated with changes in the volume and pressure of the blood in the leg.” In a freshly killed spider compression of the basal part of a leg at once extends the distal part.
A comparison of the leg musculature of the spider, the scorpion (fig. 19 G), and Limulus (fig. 11 A) shows numerous differences among the three. A common feature, however, not found in the mandibulate arthropods, is the presence of a ventral muscle or muscles in the femur extending from the lower lip of the trochanter to the base of the patella, represented by muscle 10 in Limulus, muscle 19 in the scorpion, and muscles 11, 12, and 13 in the spider (fig. 25 E).
The pretarsus of the legs of most spiders (fig. 26 C, D) has the same structure and mechanism as that of the scorpion (fig. 19 H). It is a much-shortened apical segment of the limb attached by membrane within the end of the tarsus (fig. 26 C, Ptar), and rocks on a transverse axis by the action of its antagonistic muscles attached by tendons dorsally and ventrally on its base (fig. 25 H). Usually the pretarsus is produced into a solid median claw, or dactyl (fig. 26 C, D, E, Dac), and is thus seen to represent the dactylopodite of a generalized arthropod limb. The lateral claws, or ungues (C, Un), however, are flexibly attached by basal membranes on the upper part of the pretarsus and are evidently secondary outgrowths having no independent movement of their own. In different spiders all the claws of the foot differ much in shape, and the ungues are usually armed below with teeth or comblike rows of spines (C, F).
A type of pretarsal mechanism somewhat different from that of ordinary spiders occurs in the Mygalomorphae. The pretarsus of Euryphelma, for example, is a small vertical plate without a median claw (fig. 26 A, Ptar) bearing dorsally a pair of ungues (Un). Its lower end is set in a deep median notch in the lower lip of the tarsus, and is here firmly but flexibly attached, so that it rocks back and forth on the tarsal support; its proximal movement elevates and retracts the claws (A), its distal movement protracts and deflects them (B). The usual pretarsal muscles are attached by tendons dorsally and ventrally on the base of the pretarsus, and the dorsal muscle is clearly a retractor of the claws. The tendon of the ventral muscle, however, being attached just above the tarsal fulcrum (A, B), this muscle in Eurypelma would appear to be merely an inefficient accessory to the dorsal retractor muscle. The fibers of the two muscles are closely adherent in the basitarsus (fig. 25 H), but proximally they are separated at their respective origins.
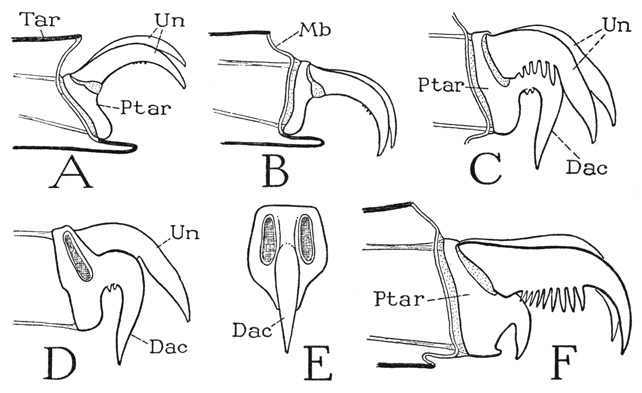
Fig. 26. Arachnida—Araneida. The pretarsus.
A, Eurypelma hentzi Chamb., pretarsus and claws retracted. B, same, pretarsus and claws protracted. C, Argiope trifasciata Forsk., pretarsus with median dactyl and lateral ungues. D, same, pretarsus with anterior claw removed, lateral. E, same, pretarsus with both lateral claws removed, end view. F, Ancylometes sp., pretarsus and claws.
For explanation of lettering see pages 126–127.
The Pedipalps
The pedipalps of the spiders have the same segmentation as the legs (fig. 27 A), but the tarsus is undivided, and the pretarsus has no lateral claws. In the male spider the pretarsus of the palpus is variously developed as a sperm-carrying and intromittent organ; in the female it is usually a simple, dactylopoditelike claw (B, Ptar), mostly concealed in the retracted condition by hairs on the end of the tarsus (C). On the base of the claw are attached the tendons (E) of the usual levator and depressor muscles of the pretarsus, the first, in the female (D, lvptar), arising in the distal end of the tibia, the second (dpptar) in the base of the tibia. The pretarsal claw of the female as seen in Eurypelma (B) is attached by membrane on the end of the tarsus and probably is protractile by blood pressure. Though it has no articulation on the tarsus, it rocks up and down because its open basal connection with the supporting membrane is shorter than the base of the claw (E), the lower angle of which is produced downward as a lever giving attachment to the tendon of the depressor muscle. The intrinsic musculature of the pedipalp is essentially the same as that of the legs; the trochanterofemoral depressor of the femur (fig. 25 D, 8) is well developed in the pedipalp, lying posterior to the other muscles in the femur.
The coxae of the pedipalps form a part of the feeding apparatus insofar as they constitute the side walls of the entrance passage to the mouth. In most of the Mygalomorphae the pedipalp coxa is produced distally mesad of the trochanter in a small coxal process (fig. 27 A, cxp), but in Atypus the coxal process is a large lobelike extension of the coxa (I, cxp), as it is in some of the other spiders (J, cxp). In a majority of the spiders, however, the coxal lobes become more differentiated and individualized structures, having the appearance of a pair of jaws appended to the coxae (F, G, cxp). The coxal lobes are commonly termed the “maxillae,” but they have no independent movement on the coxae, and according to Kästner (see Gerhardt and Kästner, 1937, ’38), the mechanical treatment of the prey is done entirely with the chelicerae, not with the coxal lobes of the pedipalps. The lobes generally bear dense brushes of hairs that curve together over the mouth entrance and serve to strain the liquid food sucked into the mouth. The term “maxilla,” therefore, is doubly inappropriate as applied to the coxal lobes of the pedipalps, because the arachnid pedipalps are appendages homologous with the mandibles of mandibulate arthropods. The coxal lobes of the araneid pedipalps, moreover, evidently do not correspond with basal lobes of the coxa, such as those of the scorpion (fig. 21 C, cxnd) and other arachnids, which have been termed coxal “endites,” since a distal coxal process (cxp) may be present also.
Fig. 27. Arachnida—Araneida. The pedipalp.
A, Eurypelma hentzi Chamb., pedipalp of female. B, same, pretarsus of pedipalp exposed by removal of tarsal hairs. C, same, undersurface of pedipalp tarsus. D, same, distal segments of pedipalp, showing pretarsal muscles. E, same, pretarsus. F, Argiope trifasciata Forsk., female pedipalps, labrum, and pedipalp sternum, anterior. G, Ancylometes sp., female, epistome, labrum, and bases of pedipalps, anterior. H, Argiope trifasciata Forsk., mesal surface of coxal lobe of right pedipalp. I, Atypus bicolor Lucas, male, prosoma and bases of appendages, ventral. J, Dysdera crocata C. Koch, female, bases of pedipalps and pedipalp sternum. K, Argiope trifasciata Forsk., sieve plate of pedipalp coxal lobe.
For explanation of lettering see pages 126–127.
Glands contained in the pedipalp coxae and their lobes are said to be present in all the Araneida; they open into the preoral cavity between the coxal lobes and are known as salivary glands, or “maxillary glands.” According to Petrunkevitch (1933), these glands are unicellular in Hypochilus, but in all other genera they are multicellular saclike organs, the number in each coxa varying with the species of spider. In Liphistius and the Mygalomorphae the glands are shown by Bertkau (1885) to be distributed along the entire length of the coxa and to open irregularly on the upper surface near the inner edge. In other spiders the glands open in a small oval area near the base of the mesal surface of the coxal lobe (fig. 27 H, svpl), known as the sieve plate because of its perforation by the duct orifices. In Argiope trifasciata the sieve plate (K) is a somewhat convex oval membrane with a dark border partly fringed with minute spines, and perforated by about 20 pores.
A male spider is usually known at a glance to be a male by the enlarged ends of his pedipalps, the terminal segments of which are elaborated into organs for the transfer of sperm to the sperm receptacles of the female. The palpal intromittent organ varies in different spiders from a relatively simple structure to one of extreme complexity, and its characters are of much importance in taxonomy for the identification of species. The segments of the limb involved are mainly the tarsus and the pretarsus, and to a lesser degree the tibia.
A relatively generalized structure of the intromittent organ is shown by Comstock (1910) and Comstock and Gertsch (1949) to be present in the genus Filistata (fig. 28 A). The organ here consists of the pretarsus alone, which is differentiated into an enlarged, subdivided basal part called the bulb and a slender, somewhat twisted terminal neck termed the embolus. The bulb arises from an alveolar depression in the end of the tarsus (Tar), which segment shows no special modification. At the apex of the embolus is the opening of an internal canal, coiled in the bulb and ending with a vesicular enlargement, which is the receptacle in which the male spider carries the sperm.
A study of the musculature of the male palpal organ leaves no doubt that the organ is the apical segment, or pretarsus, of the pedipalp, represented by the simple, dactylopoditelike claw of the female palpus (fig. 27 B, E, Ptar). As shown here in Eurypelma (fig. 28 B) the structure of the organ is even simpler than in Filistata (A); the bulb is supported on the end of the short tarsus (Tar), though it is flexed proximally, and on its base are attached the tendons of the usual two muscles of the pretarsus. The levator muscle (lvptar) arises dorsally in the base of the tarsus, the large depressor (dpptar) takes its origin in the tibia. The same structure is shown in Eurypelma californica by Barrows (1925, fig. 13), who identifies the palpal organ of the male as a hypertrophied claw, representing the dactylopodite of Crustacea. The large depressor muscle of the pretarsus in Eurypelma evidently causes a proximal ventral flexion of the intromittent organ on the tarsus. The tarsus of the male palpus, as that of the female, is a single, undivided segment; on its base is attached the usual flexor muscle of the arachnid tarsus.
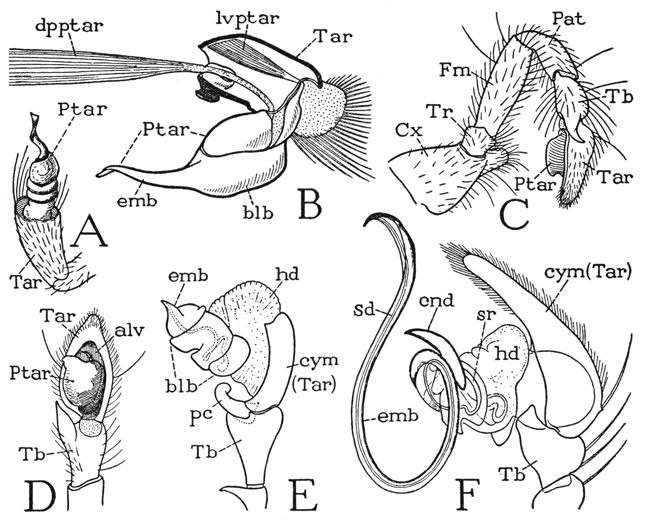
Fig. 28. Arachnida—Araneida. The male pedipalp.
A, Filistata sp., end segments of the pedipalp (from Comstock, 1910). B, Eurypelma hentzi Chamb., the pretarsal intromittent organ and section of tarsus of left pedipalp, showing pretarsal muscles. C, right pedipalp of a gnaphosid spider, posterior. D, distal segments of same, ventral. E, Erigone autumnalis Emerton, distal segments of pedipalp (from Nelson, 1909). F, Agelena naevia Walck., distal segments of pedipalp (from Petrunkevitch, 1925).
alv, alveolus; blb, bulb; cnd, conductor; Cx, coxa; cym, cymbium; dpptar, depressor muscle of pretarsus; emb, embolus; Fm, femur; hd, haematodocha; lvptar, levator muscle of pretarsus; Pat, patella; pc, paracymbium; Ptar, pretarsus; sd, seminal duct; sr, seminal reservoir; Tar, tarsus; Tb, tibia; Tr, trochanter.
In most male spiders the pedipalp tarsus itself becomes modified in connection with the pretarsus. The pretarsus shifts to the undersurface of the tarsus and takes a position near its base (fig. 28 C, D); the tarsus develops a depression, or alveolus (D, alv), for the reception of the intromittent organ and is now termed the cymbium (E, cym). Furthermore, the articular membrane between the tarsus and the pretarsus becomes enlarged in the form of a vesicle, called the haematodocha (E, hd), which may be distended by blood pressure, and is supposed to be effective in forcing the embolus into the female receptacle during mating.
The palpal organ of Erigone autumnalis (fig. 28 E), as shown by Nelson (1909), is more complex than that of the gnaphosid given at D, but it is still relatively simple. The short embolus (emb) projects from a three-part bulb (blb), which is supported on a large, inflated haematodocha (hd), and the tarsus, or cymbium (cym), has an accessory branch (pc) termed the paracymbium. An example of extreme elaboration of structure in the palpal organ is given at F, as figured by Petrunkevitch (1925) for Agelena naevia. The embolus (emb) is here a long, slender, doubly curved process containing the seminal duct (sd), and is accompanied at its base by an accessory process (cnd) termed the conductor, which in some spiders is extended the full length of the embolus. The pretarsal part of the organ, based on the haematodocha (hd), arises from a deep alveolus in the base of the elongate tarsal cymbium (cym). The palpal organ attains an even greater complexity in some other spiders, but for further examples of its variable structure the student may refer to the comparative studies by Comstock (1910) and by Osterloh (1922) or, for a concise account of the essential nature of the organ, to the paper by Nelson (1909).
Preliminary to mating, the male spider of most species spins a small, flat web on which he discharges a drop of sperm from his genital opening on the abdomen. Then, applying the tips of the palpal organs to the under surface of the web beneath the drop of sperm, the latter is taken into the sperm canals, presumably by capillary attraction. This act is called sperm induction. During mating the emboli of the male organs are inserted into the apertures of the seminal receptacles of the female, either both at the same time or alternately, and are forced into the ducts by blood pressure in the haematodochae. The ejection of the sperm is generally attributed also to blood pressure, but Osterloh (1922) suggests that the intrusion of secretion from epithelial gland cells surrounding the seminal canals may drive the sperm out, perhaps in combination with blood pressure. Numerous observations on sperm induction by the male, courtship, and mating among spiders are recorded by Montgomery (1903, 1909b); Baerg (1928) gives an account of sperm induction and mating by the tarantula; Ewing (1918) covers the life history of the house spider; Gertsch (1949) fully reviews the whole subject of courtship and mating.
The Chelicerae
The chelicerae in all spiders are two-segmented (fig. 29), and only rarely does the basal segment have a process opposing the fanglike apical segment. The appendages arise from the anterior membranous wall of the body (fig. 24 E) between the carapace above and the pedipalp coxae and labrum (Lm) below, but this supraoral position they assume secondarily during embryonic growth, as in other arachnids, from a primitive ventral position behind the mouth. In the Mygalomorphae and Liphistiomorphae, the basal segments of the chelicerae project forward from the body (fig. 24 F, G), and the fangs turn downward and posteriorly; by contrast, in the typical spiders the basal segments hang downward (fig. 29 D) and the fangs close against their mesal surfaces, where generally they are received in grooves, which may be armed on one or both margins with small spines or teeth. The fang in all cases is strongly movable by antagonistic muscles (A, B) arising in the basal segment. The chelicerae do not vary much from the typical structure, but in some of the ant spiders they attain an enormous relative length by elongation of both segments, and the basal segment is armed below with a row of slender spines (see Millot, 1949, fig. 369).
In all the araneid families but the Uloboridae the chelicerae contain poison glands. The gland is an elongate sac (fig. 29 C, E) with a duct traversing the fang to open on the convex side of the latter near the tip (C, VPr). The gland of the mygalomorph spiders is contained in the basal segment of the chelicera; in other spiders it may extend into the body cavity as far as the prosomatic nerve mass, or beyond it. The gland is covered by a layer of muscle fibers, said by Millot (1931) to be generally arranged spirally along the length of the sac (fig. 29 C) but to present variations and irregularities; in the highly venomous “black widow” spider, Lactrodectus mactans, the muscles as shown by Reese (1944) run longitudinally on the gland (E). In one spider, Scytodes thoracica (Latr.), as described by Millot (1931), the cheliceral gland is bilobed; one lobe secretes venom, the other a silk liquid which the spider ejects on its prey to entangle it before killing it with venom from the poison lobe. The chelicerae are the most essential external organs the spiders possess, since without them they could neither capture nor kill their prey.
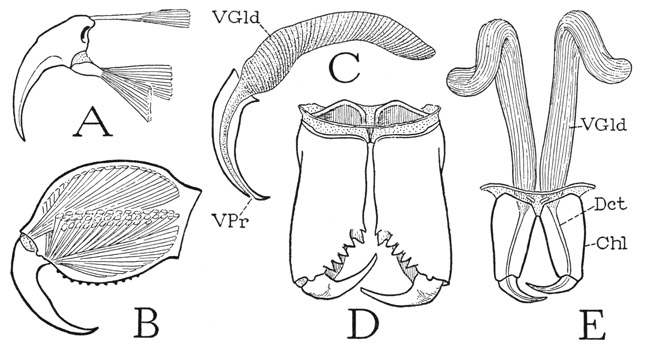
Fig. 29. Arachnida—Araneida. The chelicerae.
A, Argiope trifasciata Forsk., cheliceral fang and its muscles. B, Eurypelma hentzi Chamb., chelicera, showing muscles of the fang. C, same, venom gland of chelicera and duct. D, Ancylometes sp., chelicerae, anterior. E, Latrodectus mactans (F.), chelicerae and venom glands.
For explanation of lettering see pages 126–127.
The Eyes
The eyes of the spiders have each a single lens and are therefore of the kind known as simple eyes, or ocelli. The two median anterior eyes differ from the others in their mode of development, which turns the retinal layer upside down, and for this reason these eyes are said to be inverted.
The structure of a noninverted eye is shown at B of figure 30. Beneath the thick corneal lens (Ln) is a deep layer of translucent corneagenous cells (CgCls), which are the epidermal cells that generated the lens but which form a vitreous body in the mature eye. Beneath the corneagenous cells is the retina (Ret), composed of numerous light-sensitive cells, the nuclei of which are in the outer ends of the cells, and the sensory, receptive zones (rz) on the parts proximal to the nuclei (C, D, rz). Eyes of this kind are termed “erect” or “converted” eyes, or “prebascillary” eyes in reference to the nuclei being distal to the rodlike sensory parts of the retinal cells. A spider eye of this type is similar to the eye of a scorpion (A) except that the corneagenous cells of the scorpion eye do not intervene between the lens and the retina and the sensory zones of contiguous retinal cells form intercellular rods, or rhabdoms (Rhb). In the scorpion eye, as in the simple eye of Limulus (fig. 9 D), the retinal nuclei lie in the inner parts of the cells, as they do in the ocelli of insects.
A modification of the erect type of eye occurs in the web-spinning spiders (fig. 30 E), in which the nucleated ends of the retina cells (F) diverge from beneath the lens (E, Ret) and thus expose the receptive surfaces (rz) more directly to the light. The outer ends of the cells are imbedded in a dark pigment (Pig), and the sensory zones are surrounded by a sheath of light-reflecting cells forming a tapetum (Tap) that throws the light back into the retina.
The inverted eyes have the retinal nuclei in the inner parts of the cells (fig. 30 L) behind the light-sensitive rods (r), and are hence termed “postbascillary” eyes. In the development of these eyes, as described by Locy (1886), the prospective corneagenous layer and the retinal layer are derived from contiguous areas of the surface epidermis (H) and become superposed by an involution and consequent inversion of the retina beneath the corneagenous layer (I, J, K). The inner wall of the retinal pocket (I, K, prl) becomes a postretinal layer of the mature eye (L, prl); the retina closes against the corneagenous layer, and the opposing basement membranes, or included connective tissue, form a preretinal membrane. At the original point of involution (H) the corneagenous cells, which secrete the cuticular lens (K, L, Ln), become continuous with the epidermis (I, Epd). As a consequence of the inversion of the retina, the retinal nuclei come to be at the inner ends of the cells, while the nerve fibers issue distally; and usually the optic nerve trunk departs from one side of the eye (L, Nv). In the mature eye the outer ends of the retinal cells are extended beyond the nerve roots (M, nf) to form light-receptive, rodlike distal processes (r). In some spiders, however, the nerves are transposed to the inner ends of the cells (N, nf). In the mature eye, therefore, little evidence of inversion may remain, except for the presence of a preretinal membrane, remnants of the postretinal cell layer, and the inner position of the retinal nuclei. The eyes of the Pycnogonida are said by Wiren (1918) to resemble the inverted eyes of the Araneida.
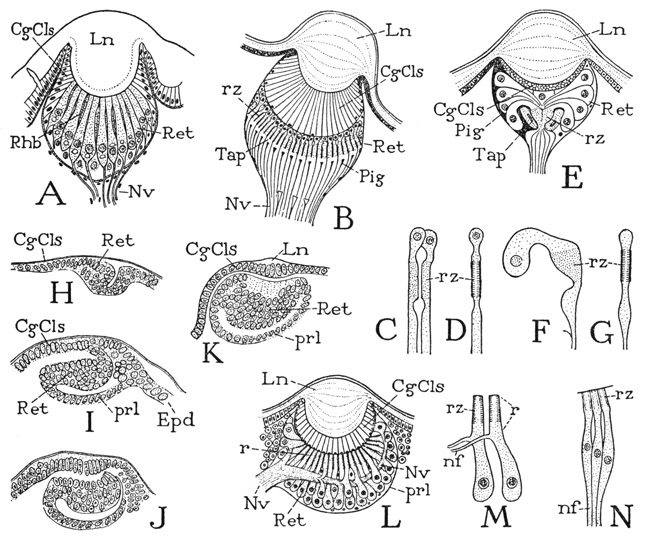
Fig. 30. Arachnida. Eyes of a scorpion and of spiders.
A, lateral eye of Euscorpius italicus (Hbst.) (from Lankester and Bourne, 1883). B, erect eye of Pardosa monticola C. L. Koch (Lycosa agricola Thorell) (simplified from Widmann, 1908). C, D, retinal cells from B. E, erect eye of Tegenaria derhamii (Scopoli) (domestica Clerck) (from Widmann, 1908). F, G, retinal cells from E. H, I, J, K, development of inverted eye of Agelena naevia Walck. (from Locy, 1886). L, inverted eye of Tegenaria derhamii (Scopoli) (from Widmann, 1908). M, retinal cells from L. N, examples of inverted retinal cells with secondarily proximal nerves (from Widmann, 1908).
CgCls, corneagenous cells; Epd, epidermis; Ln, lens; nf, nerve fiber; Nv, nerve trunk; Pig, pigment; prl, postretinal layer; r, optic rod; Ret, retina; Rhb, rhabdom; rz, receptive zone of retinal cell; Tap, tapetum.
The inverted eyes, at least in some spiders, are provided with muscles, a most unusual thing for arthropod eyes. The ocular musculature of the spiders has been described by Widmann (1908) and by Scheuring (1914). According to Widmann, in Lycosa dorsal and ventral muscles from the head wall that are attached on the eye appear to regulate the focal depth of the eye by compressing the corneagenous layer. In other species only a dorsal or a ventral muscle may be present, which, by tilting the eye up or down, changes the direction of the lens with regard to external objects. Scheuring (1914) describes in Salticus scenicus a much more complex ocular musculature, consisting of six muscles attached on each eye, to which he attributes eight different accommodation movements.
The Organs of Ingestion, Digestion, and Excretion
The spiders subsist entirely on liquid exudates extracted from the animals, mostly insects, on which they feed. The prey is seized, held, killed, punctured, lacerated, or crushed by the chelicerae, but extraoral digestion plays an important part in rendering the food available for ingestion, a highly potent digestive liquid from the stomach being discharged on or into the prey which completely liquefies the soft tissues. So copious and effective is this digestive fluid that some of the large spiders are able to consume even small vertebrates, which they kill with the venom of the chelicerae. It is probable that the salivary glands opening on the inner faces of the pedipalp coxal lobes contribute their secretion to that from the stomach.
The mouth parts of the spider, except the chelicerae, form merely a receptacle before the mouth, from which the liquid food is ingested by the sucking action of the pharynx. The preoral food chamber (fig. 31 A, PrC) is enclosed by the labrum above (Lm), the pedipalp coxal lobes on the sides, and the pedipalp sternum (IIS) below (see fig. 27 F). The coxal lobes, as already noted, are not jaws, or “maxillae” as they are often called, since they have no independent movement on the coxae; their distal ends and inner surfaces are furnished with large brushes of hairs that close over the mouth and form an effective strainer that prevents the ingress of hard pieces of the prey or food particles too large to be swallowed. The mouth (fig. 31 A, Mth) lies beneath the base of the labrum and leads directly into the sucking organ known as the pharynx (Phy). The continuity of the pharyngeal walls with the undersurface of the labrum and the upper surface of the suboral sternum might suggest that the pharynx itself is an external derivative such as the cibarial pump of sucking insects, but the arachnid pharynx is generally said to be a part of the stomodaeum. It is an elongate, flattened pouch that turns upward and posteriorly from the mouth (A, M, Phy), and the narrow oesophagus (Oe) dips downward from its upper end.
The anterior, or dorsal, wall of the pharynx is formed of a dorsal pharyngeal plate (fig. 31 A, dpi); the posterior, or ventral, wall contains a ventral plate (vpl) or pair of plates, the two being connected along the sides by flexible membranes allowing expansion and contraction. The dorsal plate is usually the more strongly developed: in the mygalomorph Eurypelma it is roundly convex ventrally (C, D), presenting a wide median lobe and a pair of lateral folds; in other spiders it is usually flat or slightly concave (F, G, I, J). Along the middle of the dorsal plate runs a narrow dorsal channel (dc), extending from the mouth to the opening of the oesophagus (C, F, J), and the lateral parts of the plate are finely and closely ridged transversely. The specific function of the dorsal channel of the pharynx is not definitely known, but Bartels (1930) suggests that the grooves between the lateral ridges of the dorsal plate direct the liquid food into the median channel, through which it is conducted to the oesophagus, while undissolved particles are retained by the ridges and grooves. The main lumen of the pharynx, then, according to Bartels, is the conduit of the digestive liquid discharged on the prey. This conclusion Bartels deduces from the observation that in spiders allowed to drink water containing a suspension of India ink or carmine particles, the particles are found in the transverse grooves and massed along the sides of the dorsal canal. The ventral plate of the pharynx may be more or less divided into lateral halves (fig. 31 E, K) and is either concave or flat according to the shape of the overlying dorsal plate.
Fig. 31. Arachnida—Araneida. The ingestion apparatus.
A, Eurypelma hentzi Chamb., longitudinal vertical section of pharynx, epistome, and labrum. B, same, epistome and labrum, anterior. C, same, dorsal plate of pharynx, ventral. D, same, cross section of dorsal plate of pharynx. E, same, ventral plate of pharynx and end of suboral sternum, dorsal. F, Ancylometes sp., dorsal plate of pharynx and labrum, ventral. G, same, cross sections of pharynx, oesophagus, and proventricular pump. H, same, oesophagus and proventricular pump. I, Argiope trifasciata Forsk., cross section of dorsal plate of pharynx. J, same, dorsal plate of pharynx, ventral. K, same, ventral plate of pharynx and end of suboral sternum, dorsal. L, Agelena naevia Walck., cross section of proventricular pump and muscles (from Brown, 1939). M, same, stomodaeum and its muscles (from Brown, 1939).
For explanation of lettering see pages 126–127.
The pharyngeal musculature of the spiders is simpler than that of most other arachnids; that of Agelena naevia, as described by Brown (1939), includes the following muscles (fig. 31 M): a pair of median dilator muscles (3) arising at the base of the labrum and converging to the front of the pharynx (in most arachnids these muscles arise on an epistomal plate above the base of the labrum, as seen at A in Eurypelma); a pair of dorsal dilators from the pedipalp coxae to the edges of the anterior wall of the pharynx; a dorsal dilator (5) from the carapace to the upper end of the pharynx; a dorsal retractor (6) from the carapace attached on the pharynx behind the last muscle; and a ventral retractor (7) from the pedipalp sternum (“labium”) to the upper end of the posterior wall of the pharynx. In other arachnids the pharynx is closely surrounded by constrictor muscles; in the spiders apparently compression of the pharynx must result from the pull of the dorsal retractor muscles.
The oesophagus is a narrow tube (fig. 31 H, M, Oe) that goes posteriorly from the pharynx through the central nerve mass to the posterior part of the prosoma, where it expands before joining the stomach to form a proventricular pump (PvP). The walls of the pump are expanded by strong dorsal muscles (L, M, dld) from an apodeme (Ap) of the carapace and by lateral muscles (dll) from the endosternum (L, Endst); compression is effected by short compressor muscles (L, cpr) on the side walls of the organ. The mesenteron section of the alimentary canal includes an axial stomach tube with diverticula, and most of the intestine. The first diverticula are a pair of tubes from the anterior end of the stomach in the prosoma, which give off lateral branches extending into the leg bases. The abdominal diverticula are mostly branched into many small lobes that form a large mass of soft tissue filling the upper part of the abdomen. Secretion and the final stages of digestion take place in the cells of the gastric diverticula. Beyond the stomach the mesenteron is continued as an intestinal tube ending with a large saclike expansion known as the cloaca, into which open the Malpighian tubules. The cloaca discharges through a very short ectodermal proctodaeum opening on the anal lobe of the abdomen.
The excretory organs of the spiders are nephidial coxal glands and the Malpighian tubules. In Liphistiomorphae and Mygalomorphae there are two pairs of coxal glands, those of the first pair opening on the first-leg coxae, those of the second pair on the third-leg coxae; the other spiders have only the pair opening on the first-leg coxae. An extensive comparative account of the arachnid coxal glands is given by Buxton (1913). The Malpighian tubules are a pair of slender tubes arising from the cloaca and branching among the stomach diverticula in the abdomen.
The Sperm Receptacles of the Female and the Epigynum
The paired ducts of the ovaries of the spiders unite anteriorly in a common part of the female genital tract, termed the uterus because it is of mesodermal origin. The uterus discharges through a short ectodermal exit passage commonly called the vagina, but termed also the “uterus externus” because in most spiders it has no copulatory function. Associated with the female genital opening are special sperm receptacles, or spermathecae. In many, mostly primitive, genera of spiders the spermathecae open directly from the vagina (fig. 32 F, Spt). There may be two, three, five, or more of these vaginal spermathecae, which are relatively simple structures. In the majority of spiders the sperm receptacles, two in number, except when secondarily divided, open independently in the neighborhood of the genital outlet, and usually the apertures are contained in a sclerotic plate on the anterior margin of the epigastric furrow known as the epigynum (fig. 24 B, Epgn). In this case, the emboli of the male intromittent palpal organs carrying the sperm are thrust into the entrance canals of the receptacles (fig. 32 G, ic), and each spermatheca (Spt) has a fertilization canal (fc) connecting it with the uterus, in which the eggs are inseminated.
The epigynum of different spiders is highly variable in size and form, in ways that have little relation to general classification but are characteristic of species. A very simple structure occurs in the common house spider, Theridion tepidariorum, in which the epigynum is little more than a basinlike impression of the abdominal wall with a sclerotic rim, containing the orifices of the intromittent canals of the spermathecae. A more typical example of the epigynum is shown here in a member of the Pisauridae (fig. 32 A), in which the epigynum is a large, strongly sclerotized plate with a pair of lateral cavities, from the inner ends of which open the spermathecal canals. A variant of this type of epigynal structure is seen in Argiope trifasciata (B) and in many other spiders. Again, however, the epigynum takes the form of a long, projecting process, as in Neoscona benjamina (C, D), with the intromittent openings on the undersurface (C, io). A reference to books and taxonomic papers on spiders will show the endless variety of forms that the epigynum assumes.
Fig. 32. Arachnida—Araneida. The female sperm receptacles and the epigynum.
A, Ancylometes sp., epigynum. B, Argiope trifasciata Forsk., epigynum. C, Neoscona benjamina (Walck.), epigynum, ventral. D, same, epigynum, lateral. E, same, sperm receptacle and intromittent duct of left side. F, Tetragnatha solandri Scopoli, vagina and connected spermathecae, internal view (from Engelhardt, 1910). G, Theridion tepidariorum (C. L. Koch), spermathecae with independent openings. H, Clubiona neglecta Cambridge (montana L. Koch), spermathecae and canals (from Engelhardt, 1910).
fc, fertilization canal; ic, intromittent canal; io, intromittent orifice; Spt, spermatheca; Vag, vagina.
The sperm receptacles connected with the epigynum in their simplest development are a pair of globular or oval, usually hard-walled vesicles at the ends of the intromittent canals (fig. 32 E). Generally, however, the organs are more complex owing to a lengthening and coiling of the canals, or also to a differentiation of the vesicles into two or more parts (G, H). An example of a highly complicated spermathecal structure is that described and figured by Petrunkevitch (1925) in Agelena naevia Walck. Here the inner ends of the intromittent canals expand into large copulatory bursae connected by ducts with the sperm vesicles, which themselves are produced into tubular diverticula, and the fertilization canals wrap themselves around the intromittent canals on their way to the uterus. A comparative study of the sperm-receiving organs will be found in the papers by Engelhardt (1910) and Osterloh (1922).
The structure of the female receptive organs and that of the male intromittent organs are intimately correlated, but it is difficult to see a reason for the great diversity in form and complexity of the copulatory apparatus. Since the structure is characteristic of species, it is sometimes explained as a “lock-and-key” device to prevent cross-breeding, though probably there are few observed cases of a male spider attempting to mate with a female not of his own species.
The Respiratory and the Circulatory Organs
The breathing organs of the araneids include book lungs like those of the scorpion, and tubular ingrowths of the integument known as tracheae. The tracheae, however, are not all of the same origin, some being primarily respiratory in function, others primarily apodemal. There are never more than two pairs of original respiratory organs, both of which may be lungs or both tracheae. They open on the venter of the abdomen and pertain to the second and third abdominal segments (segments VIII and IX). In the Liphistiomorphae, Mygalomorphae, and the Hypochilidae among the Araneomorphae both pairs of respiratory organs are lungs; in the families Caponiidae, Thelmidae, and Symphytognathidae both pairs are tracheae. In most of the rest of the araneids the organs of the first pair are lungs and those of the second pair tracheae, though in some Pholcidae tracheae are absent. The tracheal spiracles of Dysderidae, Segestriidae, and Oonopidae lie close behind the apertures of the lungs (fig. 33 D, tSp); in other families the paired tracheae open medially, sometimes near the middle of the venter of the abdomen but usually close before the spinnerets (fig. 24 B, tSp). When the spiracles have a posterior position, they lie at the sides of the sternal apodemes of segment IX, and in most such cases the hollow apodemes themselves become trachealike organs.
The position of the book lungs is generally evident because of the slightly raised and darker areas of the integument beneath them, known as the opercula (fig. 33 D, Opl). The lung openings are transverse clefts behind the opercula, those of the anterior pair being in or close to the lateral parts of the epigastric furrow (figs. 24 B, 33 D, pSp). Each aperture leads into a vertical atrial chamber (fig. 33 A, Atr), the ends of which are extended on each side beyond the spiracular cleft (B). In the anterior wall of the atrium are numerous very narrow parallel slits (A, o), which are the mouths of leaflike air pouches (lam). The lung structure of the spider differs in no way from that of the scorpion (fig. 22 D, E, F). The lung lamellae in different spiders may be vertical, oblique, or nearly horizontal, and are said to vary in number from 25 to 100 for each lung. In Eurypelma (fig. 33 C) the lamellae are triangular and are attached below on a supporting wall, so that they are free only along their dorsal edges, and it is here that the outer surfaces of adjacent lamellae are held apart by short spacing rods between them. Each lung lies in a ventral blood sinus of the abdomen, which is connected by a venous channel with the anterior part of the pericardium (I, Vp), the aerated blood being thus conveyed directly to the heart. (In most spiders the pericardial cavity around the heart is much narrower than that of Liphistius shown in the figure.)
The mechanism of respiration in spiders by the lungs has been investigated by several writers, whose work is reviewed by Kästner (1929) along with his own studies on the subject. The spiders make no pulsatory breathing movements of the body wall, and the lungs themselves have no capacity for expansion and contraction. The only lung muscle is an opener of the spiracle arising on the body wall behind the spiracle and inserted on the posterior spiracular lip. This muscle effects also an expansion of the atrium, but the closing action depends on elasticity. Kästner observes, however, that the spiracular slit is opened only when the spider has been active or is in some way artificially stimulated to activity. At other times it appears that enough air has access to the atrium to satisfy the needs of the spider. An expansion and contraction of the lung lamella was postulated by Weiss (1923) as resulting from the action of associated body muscles, other writers have attributed it to the pulsation of the blood, and it has even been supposed that the rods connecting the lamellae are elastic; but Kästner concludes that gas exchange between the atrium and the narrow cavities of the lamellae can be attributed only to diffusion. Kästner’s explanation of the respiratory mechanism of the lung books of spiders closely agrees with that of Fraenkel (1929) on the scorpion (pp. 76–77).
Fig. 33. Arachnida—Araneida. Respiratory organs and the heart.
A, diagram of a book lung of Eurypelma. B, Eurypelma hentzi Chamb., book lung, dorsal. C, same, vertical section of book lung and sublying operculum. D, Segestria senoculata L., abdomen, ventral (from Purcell, 1909). E, Miagrammops sp., apodemal posterior tracheae (from Lamy, 1902). F, Filistata capitata Hentz., nonrespiratory apodemes and small tracheal pouches of segment IX (from Lamy, 1902). G, Loxosceles rufescens L., single ventral apodeme of segment IX, and lateral tracheae opening from a common vestibule (from Lamy, 1902). H, Attus floricola C. Koch, apodemal and tracheal respiratory organs of segment IX (from Purcell, 1909). I, Liphistius malayanus Abr., pericardium and heart, anterior aorta not shown (from Bristowe and Millot, 1932).
For explanation of lettering see pages 126–127.
The tracheae of the spiders are far more variable in their structure than are the lungs. They are branched or unbranched tubes, and the inner walls of the larger trunks are strengthened by thickenings in the form of reticulations and anastomosing processes. Some writers have described the smaller tubes as having spiral taenidia in the intima, but Richards and Korda (1950) report that electron microscope studies show no circular or helical thickenings in species they examined (Theridion, Tetragnatha, Neoscona).
In the Caponiidae, one of the families having two anterior lateral pairs of tracheae but no lungs, the first spiracle on each side leads into an atrial pouch from which are given off numerous capillary tubules into the anterior blood sinus of the abdomen. The structure of these tracheae, therefore, suggests a lung in which the air pockets are tubular instead of lamelliform, and, as in the case of the lungs, it is the blood that is aerated. In those spiders in which the single pair of tracheal spiracles lies close behind the pulmonary spiracles (fig. 33 D, tSp), each spiracle opens into a wide tubular atrium from which a bundle of capillaries is given off as from the first tracheae in Caponiidae. In other spiders these spiracles have a more median and posterior position. In the Filistatidae they lie on the venter of the abdomen midway between the epigastric furrow and the spinnerets, and open into the ends of a transverse groove, or vestibulum (fig. 33 F, Vstb), laterad of the pair of ventral apodemes of segment IX (Ap); the tracheae themselves, however, are but little developed. When, as in most spiders, the spiracles are transposed to a position immediately in front of the spinnerets (fig. 24 B), the apodemes are carried back with them by the elongation of sternum IX, and the three associated structures open from a single vestibule (fig. 33 G). The apodemes may be united in a single median process as at G, but more commonly they are developed as a pair of long, tapering, hollow tubes, giving off numerous small branches (H, apTra), and thus take on the structure and supposedly the function of tracheae. United with the bases of these apodemal tracheae are the primary lateral tracheae (lTra), which vary much in their extent and branching in different spiders. Finally, in some spiders the lateral tracheae of segment IX are suppressed, and the apodemal tubes remain as the only tracheal organs (E). The conversion of hollow apodemes into respiratory organs is not confined to the spiders; in the diplopods all the tracheae arise from similar sternal apodemes serving also for muscle attachments.
Considering the fact that the araneid respiratory organs, whether lungs or tracheae, occur always on the same body segments and that either the second pair or both pairs of lungs may be replaced by tracheae, the question arises as to whether the two kinds of organs are really distinct structures, or merely different forms of development of the same thing, the ingrowths from the atrium in one case being lamellar, in the other tubular. The question has been much discussed, and both sides have been advocated by different writers, but neither side seems to offer a conclusive answer. From studies on the development of the lungs by Purcell (1909, 1910) and by Montgomery (1909a), it is evident that the lungs are intimately associated with the appendage rudiments of segments VIII and IX. The atrium of the lung is formed as a depression of the integument immediately behind the base of the appendage rudiment, and the latter itself then sinks into the cavity. According to Montgomery, the lung lamellae grow into the body cavity from the sunken part of the appendage, while the flattened external part becomes the operculum. Purcell contends that the tracheae are formed in the same way as the lungs, but Montgomery says that in the case of the tracheae the appendage rudiment disappears and the tracheae arises as an independent ingrowth behind the site of the appendage. When we note that the prosomatic spiracles of the Solpugida, Ricinulei, and Acarina are associated with the bases of the appendages, it might be deduced that the respiratory ingrowths of the Arachnida in general have a postcoxal position. The structure of the first tracheae in Caponiidae and that of the postpulmonary tracheae in Dysderidae, which are comparable to that of a lung with capillary tubes instead of hollow lamella, and the position of the single pair of tracheal spiracles in Phalangida, which is at the site of the first lungs in Araneida, suggest that the primary respiratory organs of the Arachnida, regardless of their form, are serially homologous structures. The antiquity of the scorpions gives weight to the idea that lungs have priority over tracheae, but it is a curious fact that lung spiracles have not been observed with certainty in any Paleozoic scorpion (see Petrunkevitch, 1949, pp. 134–135). There can be no question, as shown by Ripper (1931), that the arachnid tracheae have no homology with the tracheae of myriapods and insects.
The improbability of book lungs being derived from gill plates has been discussed in the section of Scorpionida. Purcell (1909) contends that the leaflets of the spider’s lung appear first as folds on the base of the associated appendage, but Montgomery (1909a) says these folds disappear and that the air pockets of the definitive lungs are formed secondarily after the insinking of the appendage rudiment. Purcell offers no explanation of how external gill plates may have been turned outside in to form internal air pouches.
The heart of the spider is a median muscular tube lying within a pericardial sinus in the dorsal part of the abdomen. The blood enters the heart through the lateral ostia; it is discharged into the abdomen through paired arteries and into the prosoma through a median aorta that divides into numerous branches. The terminal branches of the arteries and the aorta end in blood sinuses, through which the blood flows to the region of the lungs, and thence through the “pulmonary veins” to the pericardial cavity. The heart is most highly developed in Liphistius (fig. 33 I), in which there are five pairs of ostia (Ost) and five pairs of arteries (Art) besides a median posterior artery. In other spiders the heart has become shortened from behind, and the ostia reduced to four or two pairs.
The Spinnerets
Abdominal spinnerets are organs peculiar to the Araneida; they pertain to the fourth and fifth segments of the abdomen (segments X and XI). In most spiders, because of the great posterior extension of segment IX and the compression of the segments between segment XI and the anal lobe, the spinnerets come to lie just before the anus (fig. 24 B, Spn), which is at the posterior end of the body except when the abdomen takes on some unusual shape (fig. 34 K). In the Liphistiomorphae, however, a more primitive condition of the abdominal segmentation is retained, and the spinnerets arise near the middle of the venter well forward of the anal lobe (fig. 24 G).
Three pairs of spinnerets are commonly present in a compact group (fig. 24 B, Spn); their relative positions can be seen when the six are separated, as shown at D of figure 34. Two pairs are lateral, those of segment X being known as the anterior spinnerets (aSpn), those of segment XI as the posterior spinnerets (pSpn). The third pair, or median spinnerets (mSpn), lie between the bases of the posterior spinnerets. It is probable, however, that originally there were four pairs of spinnerets, since in Liphistius (A) there is a pair of slender processes between both the anterior and the posterior lateral spinnerets. In most of the spiders having six spinnerets a small conical process termed the colulus (D, Col) lies between the bases of the anterior spinnerets and is regarded as representing the first median pair of Liphistius, since in its development the colulus of some spiders is formed of two rudiments. The colulus is apparently a vestigial organ having no special function. A number of spider families lack the colulus but have in place of it a plate or pair of plates perforated by numerous silk-duct openings, known as the cribellum (I, Crb). The cribellum in its development, as shown by Montgomery (1909a), is formed from two median spinneretlike processes of the ninth segment (J, Crb), so that there is little doubt that the cribellum also is a derivative of a pair of anterior median spinnerets of more primitive spiders. The glands of the cribellum produce a special kind of gluey silk, which is combed out by a row or double row of spines on the upper surface of the basitarsus of the hind legs, termed the calamistrum. The calamistrum is thus a character of the cribellate spiders.
Fig. 34. Arachnida—Araneida. The spinnerets.
A, Liphistius birmanicus Thorell, spinnerets (from Bristowe and Millot, 1932). B, abdomen of a mygalomorph spider, ventral. C, Eurypelma hentzi Chamb., spinnerets of left side, with examples of ventral hairs and spigots. D, Argiope trifasciata Forsk., colulus, spinnerets, and anal lobe, separated. E, same, end of left anterior spinneret, mesal, and examples of spinning hairs. F, same, left posterior spinneret, mesal. G, same, spines and spigots of posterior spinneret. H, same, median spinneret. I, Filistata sp., cribellum (from Montgomery, 1909a). J, same, posterior end of embryo with rudiments of spinnerets (from Montgomery, 1909a). K, Micrathena gracilis (Walck.), abdomen, lateral. L, abdomen of male Zelotes rusticus L. Koch, ventral. M, lateral spinneret of same with spigots extruded by pressure.
For explanation of lettering see pages 126–127.
The close association of the median spinnerets with the bases of the lateral spinnerets has given rise to the idea that the spinnerets represent four biramous appendages, each composed of an exopodite and an endopodite comparable with the two rami of a crustacean limb. According to Montgomery (1909a), however, the development of the organs shows that only the larger, outer spinnerets are the true appendages of segments X and XI, the median spinnerets being merely secondary outgrowths of the body wall between the others. In most of the Mygalomorphae, which have only the two pairs of spinnerets of segment XI (fig. 34 B, C), the long, four-segmented outer spinnerets are much suggestive of a pair of legs, and the small median spinnerets are entirely unconnected with them. The lateral spinnerets are movable by muscles inserted on their basal segments, and each of the other segments is individually musculated.
A typical structure of the spinnerets in spiders having the usual three pairs is shown here in the female of Argiope trifasciata (fig. 34 D). The four outer spinnerets (aSpn, pSpn) are thick conical lobes each with a small apical segment; the two median spinnerets (mSpn) are simple triangular lobes (H), compressed from side to side. The flattened end of the apical segment of each anterior spinneret is turned somewhat mesally on the basal segment and is covered (E) with minute, upstanding cylinders bearing tapering spines, three of which are shown enlarged below in the figure. These structures are the spinning tubes of the silk glands and are known as the fusules; the gland ducts open at the tips of the spines. In a notch on the mesal margin of the segment is a much larger gland outlet distinguished as a spigot (Spg); connected with its base is a long apodeme (Ap) for muscle attachments. In some specimens there are two spigots here. The posterior spinneret (D, pSpn) is similar in shape to the anterior spinneret, but the apical segment extends up the mesal surface of the basal segment (F) and is covered with fusules somewhat longer than those of the anterior spinneret (G); on a mesal sclerotization it bears a pair of spigots (F, Spg), or in some specimens three (G). The median spinnerets of Argiope (D, mSpn, H) are fringed with small fusules on their ventral margins and have brushes of long slender ones on their expanded bases (D); near the middle of the ventral margin of each is a conspicuous spigot.
The spinnerets of different spiders differ in shape and relative size. In the male gnaphosid shown at L of figure 34 the anterior spinnerets are long and cylindrical; when one of them is compressed, six fingerlike spigots are projected from the distal end (M), from each of which a strand of silk may be drawn out. The anterior and posterior spinnerets of Liphistius (A) are multisegmented. The four-segmented outer spinnerets of Eurypelma (C) have numerous small conical spigots (two shown enlarged below) on their ventral surfaces in a dense clothing of slender, club-shaped spines. The simple median spinnerets (mSpn) have similar ventral spigots.
The silk-producing glands of the spiders are contained in the abdomen. As classified by Gertsch (1949), there are at least seven kinds of silk glands differing in size, shape, and numbers, but they do not all occur in any one spider or family of spiders. The glands that open through the small spinning tubes are the most numerous since there is a gland for each tube. These glands, either aciniform or pyriform in shape, occur in dense masses and are present in all spiders. Similar to them are the cribellar glands of the cribellate spiders. The other glands open through the spigots; they are long tubular glands, relatively few in number, cylindrical, ampullate, lobed, or branched in form.
The production of silk is a faculty peculiar to the arthropods, and one that evidently has been independently acquired in different groups. The silk glands of the pseudoscorpions are in the chelicerae and discharge through the movable fingers of these appendages. Among the diplopods silk glands are present in the Nematophora opening through spines on the end of the body; in Symphyla silk glands open through the abdominal cerci. The silk glands of insects are mostly labial glands, but the Embioptera have silk glands in the fore tarsi, and in certain beetles and neuropterans the Malpighian tubules produce silk discharged through the anus. For quantity production the lepidopterous silkworms probably rank first, but for architectural achievements in the art of spinning the spiders excel all their arthropod relatives.
A TICK
The ticks and the mites constitute the arachnid order Acari, or Acarina. The number of species, genera, and families is so large that acarologists find it necessary to divide the order into six or more suborders, and under each suborder to recognize several subsidiary ranks, one under the other, before coming even to the families (see Vitzthum, 1931, 1940–1943). An important character used in classifying the larger groups is the position of the spiracles, or the presence or absence of spiracles. The ticks belong to the acarine group called the Ixodides, and most of them are included in two families, the Ixodidae and the Argasidae.
In external appearance the Acarina have little resemblance to other arachnids, but fundamentally in both their outer and their inner organization they show that they are merely arachnids that have developed a highly specialized type of structure, mostly correlated with parasitic habits. Two distinctive acarine characters are the absence of any constriction or other separation between the prosoma and the opisthosoma (fig. 35 A) and the presence of a discrete head structure (Capt), known as the capitulum, or gnathosoma. As in other arachnids, the adult has six pairs of appendages, including a pair of chelicerae, a pair of palps representing the pedipalps, and four pairs of legs. The food is generally a liquid, either blood or body juices extracted directly from the host, body tissue liquefied by predigestion, or the sap of plants. Some species of mites, however, feed on dry material, and ingest spores or particles of the food, but it is questionable whether they swallow such material in a dry form or suspended in an ejected liquid. The chelicerae are the cutting or piercing instruments, the organ of ingestion is a sucking pharynx as in other arachnids.
For a study of the structure of a tick we may take the common “dog tick,” Dermacentor variabilis (Say), of the eastern and central states, a member of the family Ixodidae. It is closely related to the western Dermacentor andersoni Stiles, and both species carry the Rickettsiae of Rocky Mountain spotted fever. Other common genera of the same family are Ixodes, Amblyomma, Boophilus, Ripicephalus, and Haemaphysalis. The ticks are blood feeders at all stages of their postembryonic development. From the egg hatches a six-legged young tick called the larva, or seed tick; it feeds on small rodents, and when replete with its first meal it drops off the host and undergoes a moult into a second-stage form known as a nymph, which has eight legs. The nymph, after feeding usually on some larger mammal, drops off and moults into the adult. In the tick embryo rudiments of eight legs are present, but those of the fourth pair, as described by Falke (1931) in Ixodes ricinus, are reduced to small masses of cells that remain latent beneath the cuticle of the larva and redevelop into the fourth pair of functional legs during the quiescent stage that precedes the moult to the nymph after the larva has engorged. The reappearance of the fourth pair of legs in the nymph after their suppression in the larva, therefore, is not literally a case of reacquisition of a lost organ.
The active nymph, which resembles the adult female but is much smaller, finds a suitable animal, usually a field mouse, to which it attaches itself, and fills with blood in from four to eight days. It then detaches from the host, and, after a few days of activity, the skin splits and an adult tick, either a male or female, crawls out. The adults find a new host, almost any common mammal, including man, and engorge themselves with blood. An unfed female of Dermacentor variabilis, measuring about 5 mm. in length, after feeding becomes an oval, turgid bag 13 mm. long and 10 mm. wide, with a thickness of 5 mm. The increase in body size is accompanied by the flattening out of innumerable small wrinkles in the denser, outer layer of the cuticle, and probably by a stretching of the soft inner layer. The male tick does not become distended with feeding, but the body thickens slightly. Mating takes place on the host, and the fully fed female drops off to lay her eggs on the ground. There is no fixed relation between the developmental stages of the tick and the yearly seasons, as there is in most insects; overwintering stages of ticks may include adults, nymphs, and larvae, or even eggs. The results of an elaborate study of the seasonal stages of the dog tick on the island of Martha’s Vineyard, Massachusetts, are given by Smith, Cole, and Gouck (1946).
General Structure of the Body— The body of an unfed Dermacentor variabilis (fig. 35 A) is ovate in outline, much flattened dorsoventrally, and deeply incised anteriorly for the reception of the capitulum (Capt). The broadly rounded posterior end is margined with 11 quadrate divisions of the integument termed festoons (fst). A female (A) is readily distinguished from a male by the presence of a shieldlike area on the anterior part of the dorsum, called the shield, or scutum (Shld), which is distinctly defined by a thickened margin and is variously mottled with white pigment. The male has no such shield, or, as it is said, the shield covers his entire back. Located laterally on the dorsum just behind the second legs, in the lateral angles of the shield of the female, is a pair of ocellar eyes (O). Near the middle of the back in each sex, shortly behind the shield of the female (fig. 35 A), are two clear, circular areas, each with a small central body bristling with minute peglike processes, presumably sensory in function. These organs are larger in the female than in the male. The integument otherwise is spotted with the alveoli of very short, blunt setae directed forward, and is everywhere speckled with cuticular pores said to be the outlets of dermal glands. In the unfed female tick the surface of the integument, except that of the shield, is marked by fine, closely set, parallel striations running in highly irregular, zig-zag bands from one side to the other. These striations are the minute wrinkles seen in sections on the surface of the cuticle; they entirely disappear with the stretching of the skin in the engorged female. The shield of the female, which gives attachment to the leg muscles, remains unaffected by the increase in size of the body. The cuticular striations are not present in the male. The surface features of the integument are best seen by cutting the dorsal and ventral walls apart, so that they can be examined individually by transmitted light.
Fig. 35. Arachnida—Acarina. General external structure of a tick.
A, Dermacentor variabilis (Say), adult unfed female, dorsal. B, same ventral. C, same, left spiracle and surrounding plate. D, Ixodes reduvius L., section of a large vacuole of spiracular plate, with apparent sense organ in basal canal (from Nordenskiöld, 1909). E, Dermacentor variabilis (Say), male, details of undersurface. F, same, third left leg of female, ventral. G, same, tarsus and pretarsus with empodium spread out, dorsal. H, same, optical section of distal part of first leg, showing tarsal sense organs and pretarsal muscles. I, same, tarsus and pretarsus of third leg as seen by transmitted light.
For explanation of lettering see pages 126–127.
On the underside of the body (fig. 35 B) the flat coxal segments of the legs form a double row of lateral plates, separated by a wider median space in the female (B) than in the male (E). Between the coxae of the second pair is the gonopore (Gpr), a V-shaped aperture in the female (B), a transverse slit in the male (E). From the sides of the gonopore two lines, the genital grooves, run backward and widely diverge behind the fourth coxae to the posterior margin of the body. In the male (E) the parts of the grooves between the coxae are usually farther apart than in the female. Although the genital opening of the tick appears to be on the prosomatic part of the body, it is probable that the abdominal sterna have been extended forward between the legs and that the gonopore is really on segment VIII as in other arachnids. The anus (B, E, An) lies at about the center of the undersurface of the abdominal region of the tick, but, as with the spiders, its position represents the posterior pole of the body.
The Spiracles— The tick has only a single pair of spiracles (fig. 35 B, Sp), which in Dermacentor lie laterally on the venter of the abdominal region behind the last legs. Each spiracle of Dermacentor is contained in a large spiracular plate, or peritreme (C). The surface of the plate is smooth, but it has a finely punctate appearance, and in transmitted light the punctations appear as minute bright spots of two sizes. Sections show that the interior of the plate is vacuolated by larger and smaller cavities corresponding to the bright spots seen on the surface. The small vacuoles lie in a middle layer of the plate between cuticular strands that connect a thinner outer layer with a thick inner layer of the plate. The large vacuoles extend to the outer surface, where they are said to have minute openings, and they open on the inner surface of the plate through narrow basal canals. The large vacuoles of Dermacentor are described by Stiles (1910) as goblet-shaped, the stems being the basal canals; those of Haemaphysalis punctata are said by Nuttall, Cooper, and Robinson (1908) to be pear-shaped; in Ixodes reduvius they are shown by Nordenskiöld (1909) as more slender cavities (fig. 35 D, Vac); and Falke (1931) describes those of Ixodes ricinus as tubular pores opening externally on the surface of the plate and internally into the wider basal canals. Both Nordenskiöld and Falke find within the basal canals structures that appear to be sense organs (D), each, according to Nordenskiöld, being connected with a sense cell (SCl), or sometimes with two cells, in the sublying epidermis. On the other hand, Mellanby (1935) says the spiracular plate of Ornithodoros moubata is pierced by minute pores opening into the spiracular atrium, and in Dermacentor andersoni Douglas (1943) asserts that the atrium is surrounded by a periatrial space with which the goblet vacuoles are connected, “thus establishing a passage for air via the area porosa.” Yet Douglas notes that the stems of the goblets (opening through the inner wall of the cuticle) “contain protoplasmic strands from the hypodermis.” The structure of the spiracle plate clearly needs further study. The plate, however, must have some function in connection with respiration; if it is sensory, Falke suggests that it possibly gives the tick a warning to close the spiracles against some harmful condition of the air. Stiles (1910) gives a large number of illustrations showing the form of the spiracular plates and their surface appearance in species of Dermacentor. The spiracular orifice is a small crescentic slit in an elongate oval depression of the plate; it opens into an atrial chamber, from which branching tracheae are given off to all parts of the body.
The Legs— The legs of the tick have each eight segments (fig. 35 F). The coxae, as already noted, have the form of plates on the undersurface of the body (B); those of the first legs are slightly movable, but the others are adnate on the body wall, and the principal movement of all the legs is at the coxotrochanteral joints. A very small second trochanter (F, 2Tr) is firmly fixed to the base of the femur in each leg. The next three segments, the femur, patella, and tibia, are of approximately equal length. The tarsus (Tar) is differentiated into a thick, strongly sclerotic proximal section of two subsegments and a slender, delicate distal part that forms a stalk supporting the pretarsal foot (Ptar). The footstalk itself is divided into three subsegments (I), the short basal one being mostly retracted into the end of the preceding section of the tarsus, which, in all the legs but the first, is produced into a strong, posterior, hooklike process.
The pretarsus is a small sclerotic body (fig. 35 G, I, Ptar) articulated on the end of the footstalk. Dorsally it bears a pair of long, slender decurved claws (I, Un), and ventrally a large pad (Emp), which when spread out (G) has an oval outline and a flat, smooth undersurface. The pad is evidently an adhesive organ. To be consistent with insect nomenclature, the footpad of the tick must be termed a pulvilliform empodium, unless it is composed of two lateral parts united, “pulvilli,” so called, being paired lobes beneath the bases of the claws. In some Acarina the body of the pretarsus is produced into a median claw between the bases of the two articulated lateral claws, or ungues, while in others there may be only a median claw. On the base of the pretarsus are attached the tendons (I, lvt, dpt) of the usual two pretarsal muscles, the levator (H, lvptar) arising on the dorsal wall of the proximal part of the tarsus, the depressor (dpptar) arising mostly in the tibia but with a small bundle of fibers from the base of the tarsus.
On the tarsi of the forelegs of the ticks are located important sense organs (fig. 35 A, SO), known as Haller’s organs from their discoverer. At the distal end of the basal subsegment of the tarsus, on the dorsal surface, is a capsulelike cavity with only a small opening to the exterior, containing a number of sensory setae (H, SO). Just beyond it on the base of the second subsegment of the tarsus is a shallow, open depression also containing sensory setae. Each group of setae is innervated from its own sublying sense cells. The structure of the organs has been described by Schulze (1941) and by Lees (1948). The capsular organ is said by Schulze to be fully developed in all ticks, the open organ is variable and may be absent in some species. It has long been known that the capsular organ is an odor receptor; experimental evidence presented by Lees suggests that the open organ is responsive to humidity. Probably by means of these tarsal organs the ticks recognize their prospective hosts. When a tick has ascended a blade of grass or the stem of a bush, it clings to the support by the third legs, keeping the others free for grasping, while the long first legs are extended to catch the odor of a passing animal. The sensory reactions of ticks have been described by Totze (1933) and by Lees (1948).
The Capitulum and the Organs of Feeding— The headlike capitulum is the most distinctive feature of the Acarina; in the ticks it is a strongly sclerotized structure (fig. 36 A, B), with a thick base bearing laterally a pair of palps and produced medially into a cylindrical rostrum directed forward. The basal part, or basis capituli (A, Bcp) as it is called, has particularly strong walls and is rectangular in cross section; a narrower, posterior necklike extension fits into the receptive cavity of the body between the bases of the first legs, where it is attached by a tough, flexible integument. On the dorsal surface of the basis capituli of the female are two porous areas, the areae porosae (fig. 35 A, apr), which, according to Falke (1931), contain sense organs like those of the spiracular plates. The rostrum is composed of three parts: dorsally are two closely adjacent tubular extensions of the basis (fig. 36 A, cSh), which ensheath the long slender chelicerae (C, Chl); ventrally (B) a prolongation of the lower wall of the capitulum forms a long, somewhat spoon-shaped underlip, known as the hypostome (Hst), armed in D. variabilis with six rows of strong teeth on its undersurface. Above the base of the hypostome is the mouth (C, Mth), which leads into the pharynx (Phy). Projecting over the mouth, beneath the cheliceral sheaths, is a conical lobe ending in a slender, tapering stylet, which is the labrum (Lm). On the dorsal surface of the hypostome a narrow median groove, or gutter, runs back beneath the labrum to the mouth and is the food conduit (fc) leading into the pharynx, which is the sucking organ. The distal end of the hypostome (B) is delicately membranous and is covered by numerous minute papillae bearing short hairs, presumably having a sensory function. The rest of the hypostome is rigid, and its teeth are directed toward the base. The chelicerae are the cutting organs that make an incision into the skin of the host; the hypostomal teeth retain the rostrum when the latter penetrates the wound.
The palps are movably attached to the basis capituli at the sides of the rostrum (fig. 36 A, B, Plp). In Dermacentor variabilis they extend somewhat beyond the end of the rostrum, and each is four-segmented, though the basal segment is firmly united with the second. The apical segment is a very small lobe bearing sensory hairs, located in a membranous ventral area on the end of the third segment (B). Dorsally each palp is expanded mesally in a broad flange that, in the usual position of the palps, overlaps the upper surface of the rostrum. When, during feeding, the rostrum penetrates the skin of the host, the palps spread out to the sides.
The chelicerae are long, slender, cylindrical rods with their bases deeply sunken into the upper part of the capitulum and, when fully retracted, extending into the anterior part of the body (fig. 36 C, Chl). The shaft of each organ is indistinctly divided into two segments and bears at the distal end a small, movable apical segment. The base of the apical segment (H) is produced into an elongate, toothed mesal process and bears a shorter, strongly toothed, movable lateral process. On the base are attached the tendons of two antagonistic muscles arising proximally in the shaft; in action the apical segment as a whole turns laterally and somewhat downward. The lateral dentate lobe of the apical segment of the tick’s chelicera is clearly not the “movable finger” of the chelicerae of other arachnids, since the tendons of both muscles are attached on the common base of the segment. The entire segment, therefore, appears to be the “movable finger” with an accessory lateral lobe. It is guarded mesally by a liplike extension of the end of the shaft, and, covering the toothed processes dorsally, is a delicate membranous fold (H, I). The chelicerae can be protracted from their sheaths and retracted; retraction is produced by muscles attached on the cheliceral bases, protraction is thought to be produced by a bulblike contraction of the body.
The cheliceral sheaths are tubular extensions of the capitular integument that entirely surround the retracted chelicerae (fig. 36 C). Their dorsal walls are weakly sclerotized, the ventral walls are membranous. At the distal end the outer wall of each sheath (ocSh) is inflected to form an inner membranous tube, or inner sheath (icSh), that more closely invests the cheliceral shaft and is attached on the proximal segment of the latter. The free part of the inner sheath becomes everted with the protraction of the chelicera. On the basal part of the rostrum the outer sheaths are united (A, cSh), and their cavities are separated by only a median septum.
In the more generalized Acarina there is a free epistomal region proximal to the labrum, beneath the chelicerae, on which arise dorsal dilators of the pharynx. In the argasid ticks as shown by Robinson and Davidson (1913–1914) and by True (1932), and in Ixodes ricinus as described by Arthur (1946), a similar plate, termed the subcheliceral plate, lies beneath the chelicerae and gives attachment to the dorsal dilator muscles of the pharynx. The subcheliceral plate of these ticks, therefore, must be the epistome. In Argas persicus and Ixodes ricinus the plate is thin medially but thickened along the margins (fig. 36 F, Epst) where the dorsal muscles of the pharynx (dld) are attached on it. With the plate apparently is united the lower walls of the outer sheaths (cSh) of the chelicerae. In Dermacentor a distinct epistomal sclerotization extends a short distance proximally from the upper side of the base of the labrum, but otherwise the epistome (C, Epst) is not distinguishable from the under walls of the cheliceral sheaths. In Dermacentor there are no dorsal dilator muscles of the pharynx.
Fig. 36. Arachnida—Acarina. The capitulum and mouth parts.
A, Dermacentor variabilis (Say), capitulum, dorsal. B, same, capitulum, ventral. C, same, diagrammatic longitudinal section of capitulum, reconstructed from dissections. D, same, pharynx and labrum, dorsal. E, same, cross section of pharynx and its muscles. F, Argas persicus (Oken), transverse section of capitulum through pharynx (from Robinson and Davidson, 1913–1914, relettered). G, Opiliacarus segmentatus With, Notostigmata, capitulum, lateral (from With, 1904). H, Dermacentor variabilis (Say), apical segment of left chelicera, ventral. I, same, apical segment of left chelicera, dorsal.
For explanation of lettering see pages 126–127.
The extraoral space between the bases of the cheliceral sheaths and the hypostome has been called the “buccal cavity” of the tick, but since it is entirely outside the mouth it is properly a preoral cavity. The ducts of the salivary glands, one on each side (fig. 36 C, SlDct), open into a pocket of the preoral cavity (Slv) above the base of the labrum.
The pharynx of Dermacentor is an elongate wedge-shaped sac (fig. 36 D, Phy) lying in the ventral part of the capitulum (C). In the contracted condition, as seen in cross section (E), it is triradiate, being very narrow above and expanded ventrally, with the four walls incurved toward the lumen. On each of the side walls are attached about seven winglike bundles of dilator muscle fibers (dll), and on the ventral wall a double row of ventral dilators (dlv), all of which arise on the walls of the capitulum. Between the four angle ridges of the pharynx are stretched small constrictor muscles alternating with the bundles of dilator fibers. In most other Acarina that have been studied, dorsal dilator muscles of the pharynx arise on the epistomal plate, as shown in the argasid tick Argas persicus (F, dld) by Robinson and Davidson (1913–1914). In Argas and Ixodes, however, the dorsal wall of the pharynx is wide, and the ventral wall narrow. Douglas (1943) records the presence of only lateral and ventral dilators of the pharynx in Dermacentor andersoni, and in D. variabilis the writer has observed no muscles attached on the narrow upper wall of the pharynx. The anterior end of the dorsal pharyngeal wall in Dermacentor is produced into a pair of lateral plates (D). Douglas has shown that transverse muscles on the dorsal surfaces of these plates apparently serve to close the mouth by depressing a valvelike lobe of the pharynx lumen between them. From the inner end of the pharynx the slender oesophagus (C, Oe) proceeds back to the stomach.
A more detailed description of the structure of the capitulum and the feeding mechanism of Ixodes hexagonus Leach is given in a recent paper by Arthur (1951a).
At the base of the dorsal wall of the capitulum of the female tick, in the anterior end of the body, is an eversible wax-secreting structure known as the organ of Géné. It is used for coating the eggs with a protective covering of wax. The structure of the organ in Ixodes ricinus and the method by which the female tick brings the eggs into contact with it are fully described by Lees and Beament (1948) and by Arthur (1951a). At the time of oviposition the capitulum is turned downward and backward against the undersurface of the body in front of the gonopore. The vagina, containing an issuing egg, is then everted in the form of a tube, and the egg is pressed against the organ of Géné, which, when fully extruded, has a globular form with a pair of bifid horns on its outer end. Finally, the organ is retracted, leaving the egg on the hypostome, and the capitulum reverts to its usual position. Most eggs that are not waxed shrivel quickly, and “few hatch even in a humid atmosphere.”
Inasmuch as no other arachnid has a head structure comparable with the capitulum of the Acarina, it becomes somewhat of a problem to understand how the acarine capitulum has been evolved from ordinary arachnid parts. The generalized notostigmatid mite Opiliacarus (fig. 36 G), as figured by With (1904), however, readily gives the clue to the capitular composition. The chelicerae (Chl) of Opiliacarus are fully exposed, except at their bases, which are slightly retracted into the capitulum. The large pedipalp coxae (pdpCx) are united below and extended upward on the sides to a small dorsal plate (Tect) over the cheliceral bases. It is clear, then, that a union of the dorsal plate with the upper ends of the coxae would produce an annular basis capituli. Distally the united coxae are produced into a small hypostome (Hst), and their upper margins beneath the chelicerae are united by an epistomal bridge giving attachment to dorsal dilator muscles of the pharynx. In the formation of the notostigmatid capitulum, therefore, the only part added to the usual arachnid structure is the dorsal plate connecting the upper ends of the pedipalp coxae. This plate can be nothing more than a secondary sclerotization of the integument over the bases of the chelicerae, which in the ticks is produced into the cheliceral sheaths. Acarologists commonly call the dorsal wall of the capitulum, together with the upper walls of the cheliceral sheaths, either the “rostrum” or the “epistome.” The term “epistome” in this connection is clearly misapplied. Since the surface in question is the roof of the basis capituli, the writer (1948) has named it the tectum capituli (fig. 36 G, Tect). The functional rostrum of the tick is the composite feeding organ composed of the cheliceral sheaths, the chelicerae, and the hypostome.
Explanation of Lettering on Figures 17-27, 29, 31, 33-36
aAp, anterior apodeme of coxa.
ab, abductor muscle.
ad, adductor muscle.
An, anus.
anL, anal lobe.
Ap, apodeme.
apr, area porosa.
apTra, apodemal trachea.
Art, artery.
aSpn, anterior spinneret.
Atr, atrium of spiracle.
b, sternal articulation of coxa.
Bcp, basis capituli.
bl, book lung.
BW, body wall.
Capt, capitulum.
Chl, chelicera.
chlF, cheliceral foramen.
clg, cardiac ligament.
Col, colulus.
Cp, carapace.
cpr, compressor muscle.
Crb, cribellum.
cSh, cheliceral sheath.
Cx, coxa.
cxnd, coxal endite (1cxnd, of first leg; 2cxnd, of second leg).
cxp, coxal process.
cxR, coxal ridge.
cxs, coxal sulcus.
Dac, dactyl, median claw of pretarsus.
dc, dorsal canal of pharynx.
Dct, duct.
dld, dorsal dilator muscle.
dll, lateral dilator muscle.
dlv, ventral dilator muscle.
dmcl, dorsal muscle.
dpl, dorsal plate of pharynx.
dpptar, depressor muscle of pretarsus.
dpt, tendon of depressor muscle.
eAp, epistomal apodeme.
Emp, empodium.
Endst, endosternum.
epgF, epigastric furrow.
Epgn, epigynum.
Epst, epistome.
fc, food canal.
Fm, femur.
fst, fastigium.
Gld, venom gland.
GO, genital opening.
Gpr, gonopore.
Gtr, gonotreme.
h, dorsal hinge of tarsus on tibia.
Hst, hypostome.
Ht, heart.
icSh, inner cheliceral sheath.
L, leg.
lam, lamella of lung.
Lm, labrum.
lmcls, longitudinal muscles.
lTra, lateral trachea.
lvptar, levator muscle of pretarsus.
lvt, tendon of levator muscle.
Mb, membrane.
mcl, muscle.
mSpn, median spinneret.
Mth, mouth.
o, atrial openings into lung lamellae.
O, ocellus.
ocSh, outer cheliceral sheath.
Oe, oesophagus.
Opl, operculum.
Ost, ostium of heart.
pAp, posterior apodeme of coxa.
Pat, patella.
Pcrd, pericardium.
pdpCx, coxa of pedipalp.
pdpF, foramen of pedipalp.
Pec, pecten.
pgf, pregenital fold.
Phy, pharynx.
Pl, pleural fold.
Plp, palp.
PrC, preoral food cavity.
pSp, pulmonary spiracle.
pSpn, posterior spinneret.
Ptar, pretarsus.
pv, articular pivot.
PvP, proventricular pump.
Rect, rectum.
s, septa between openings of lung lamellae.
S, sternum.
SCl, sensory cell.
Shld, shield of female tick.
SlDct, salivary duct.
Slv, salivary pocket.
SO, sense organ of tarsus.
Sp, spiracle (1Sp, first spiracle; 2Sp, second spiracle).
Spg, spigot.
Spn, spinneret.
Stn, sting.
svpl, sieve plate, opening of salivary glands.
t, tendon.
T, tergum.
tar, tarsomere (1tar, 2tar, first and second tarsomeres).
Tar, tarsus.
Tb, tibia.
Tect, tectum capituli.
tmcl, transverse muscle of labrum.
tp, tarsal process.
Tr, trochanter.
tSp, tracheal spiracle.
u, tarsal articulation of pretarsus.
Un, unguis, lateral claw of pretarsus.
Vac, vacuole.
VGld, venom gland.
vmcl, ventral muscle.
Vp, vena pulmonaris.
vpl, ventral plate of pharynx.
VPr, venom pore.
Vstb, vestibule.