LIMULUS
LIMULUS, and its living relatives generally assigned to two other genera, are the animals known as “horseshoe crabs” or “king crabs.” They, together with various fossil forms, constitute the class Xiphosurida of the subphylum Chelicerata. Limulus polyphemus Latr., also known under the generic name of Xiphosura, lives along the Atlantic coast of North and Central America; other species are found in the waters of Asia, Japan, and the East Indies. The xiphosurids for the most part inhabit fairly shallow water over sandy bottoms along the ocean shores, but the Asiatic species, Carcinoscorpius rotundicauda Latr., is said to live in brackish estuaries, or even in rivers where the water is practically fresh (see Annandale, 1909). The horseshoe crab is an animal that adds romance to zoology; it is a relic of past ages that now stands alone in the midst of creatures of later origins and of more modern types of structure. The ancestors of the horseshoe crabs were companions of the trilobites.
General External Structure
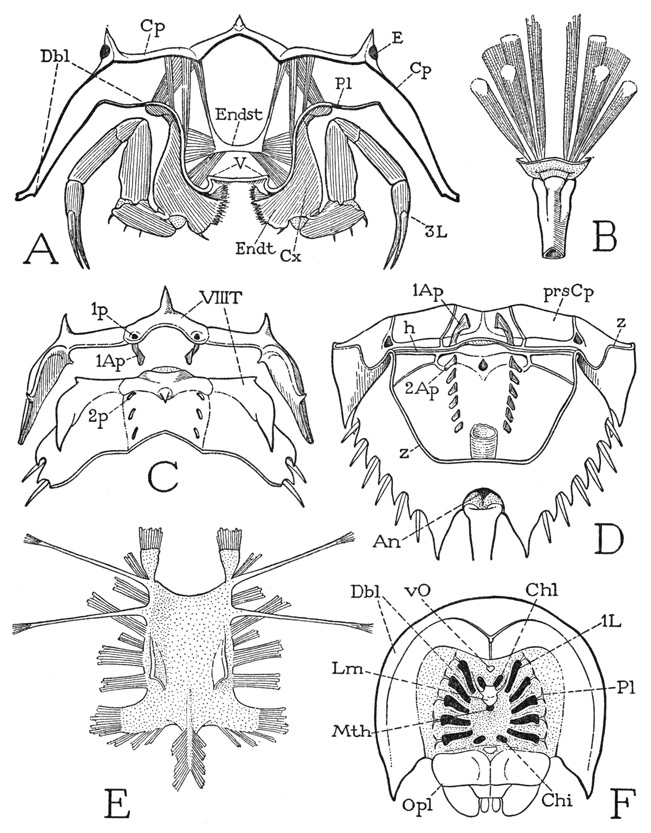
The body of Limulus (fig. 5 A) is distinctly divided into an anterior prosoma and a posterior opisthosoma, the second part being freely movable up and down on the first; the opisthosoma carries a long, strong, independently movable tail spine. The animal gets its common name from the shape of the prosoma, which is covered by a convex dorsal shield, or carapace, in outline suggestive of a horseshoe. The opisthosoma is hexagonal, with its broad base attached to the prosoma on a strong transverse hinge between the projecting posterior angles of the prosomatic carapace. The lateral margins of the opisthosoma are indented by six notches on each side, in which are seated slender movable spines; the tail spine arises from a deep indentation of the posterior margin. The body of the adult animal is entirely unsegmented.
The dorsal surface of both the prosoma and the opisthosoma is elevated along the middle (fig. 5 A), and on the broad lateral areas of the prosomatic shield is a pair of compound eyes (E) at the sides of longitudinal ridges. In a general way, therefore, in its dorsal aspect, Limulus has a rather striking resemblance to a trilobite, except for the absence of segmentation in the opisthosoma and the presence of the tail spine. Six small pits along each side of the median elevation of the opisthosoma of Limulus, however, probably mark the lines of union between primitive segments of the opisthosoma corresponding with the lateral spines. In Limulus there is a pair of very small simple eyes (dO) situated on the sides of a median spine on the anterior part of the prosoma, but in some trilobites a sense organ of some kind is present in the same position.
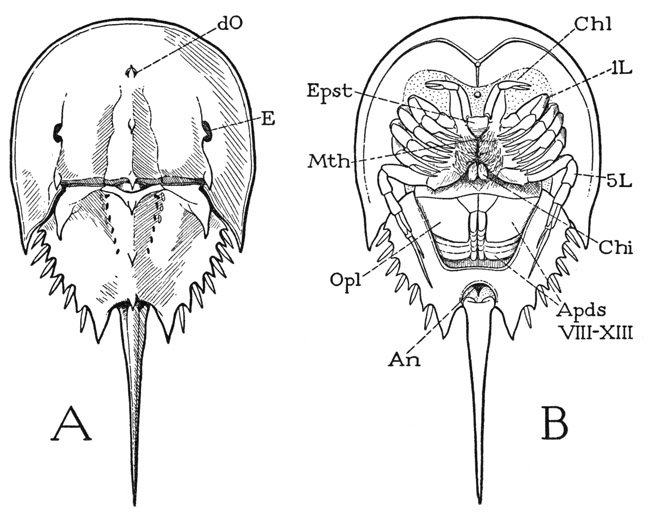
Fig. 5. Xiphosurida. Limulus polyphemus L., dorsal (A) and ventral (B).
An, anus; Apds, opisthosomatic appendages; Chi, chilarium; Chl, chelicera; dO, simple dorsal eye; E, compound lateral eye; Epst, epistome; L, leg; Mth, mouth; Opl, operculum.
On the undersurface of Limulus (fig. 5 B) the appendages are most in evidence, but the xiphosurids in common with the arachnids have no antennae. On the prosoma are six pairs of jointed, leglike limbs, and on the opisthosoma six pairs of broad, flat lobes projecting posteriorly and underlapping each other, so that the posterior five are almost covered by the first. In the differentiated form and function of the appendages, and in the lack of antennae, the xiphosurids differ conspicuously from the trilobites. The prosomatic limbs include an anterior pair of small appendages termed the chelicerae (Chl), which are directed forward, and five pairs of legs (L) extended laterally. Behind and between the bases of the last legs are two small spiny lobes (Chi) known as the chilaria, which represent a seventh pair of prosomatic appendages. The legs are attached by their dorsoventrally elongated basal segments on the sides of a deep axial part of the body (fig. 7 A), and the margins of the prosomatic carapace are reflected ventrally to the leg bases as a broad doublure (Dbl). In cross section, therefore, Limulus again resembles a trilobite (fig. 4 A); the body of the animal in each case is a cylinder containing the principal viscera, with wide flat expansions of the dorsum extending over the limbs.
If the legs are spread apart (fig. 6 A), between their bases is exposed a median triangular area of the ventral integument, which is the sternal region of the prosoma. Anteriorly at its apex is the mouth (Mth), which has a central position beneath the prosoma (fig. 5 B). If the prosomatic appendages are removed from a specimen (fig. 7 F), it will be seen that the bases of the chelicerae and legs are radially arranged around the median sternal area, the chelicerae being directly in front of the mouth and the first two pairs of legs diverging forward from its sides; the chilaria (Chi) block the posterior end of the space between the legs. With the appendages removed, the doublure (Dbl) is fully exposed. Its peripheral part forms a wide horseshoe-shaped area, which has the hard, horny texture of the dorsal surface of the carapace and slopes steeply upward (A). The inner part immediately surrounding the bases of the appendages takes a horizontal position and becomes membranous (F), but it contains on each side a series of five small Y-shaped sclerites (Pl), to the stems of which the leg bases are specifically articulated (fig.6 C). These sclerites, the “coxal pivots” of Benham (1885), clearly are to be regarded as pleurites, since, in that they support the appendages, they correspond with the plates called pleura in most other arthropods. On the membranous part of the doublure of Limulus before the bases of the chelicerae is a median papilla (fig. 7 F, vO), the supposed “ventral eye.” Supporting the chelicerae is a small epistomal plate (fig. 6 B, Epst), which bears the labrum (Lm) projecting beneath the mouth (fig. 7 F).
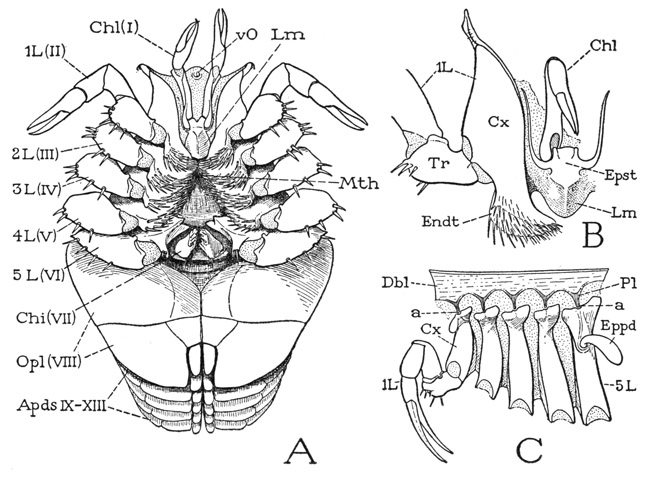
Fig. 6. Xiphosurida. Limulus polyphemus L.
A, the appendages in place on the body, ventral. B, epistome, labrum, right chelicera, and base of right first leg, anterior. C, coxae and first leg of left side, with pleural articulations.
a, pleural articulations of coxae; Apds, opisthosomatic appendages; Chi, chilarium; Chl, chelicera; Cx, coxa; Dbl, doublure; Endt, coxal endite; Eppd, epipodite; Epst, epistome; L, leg; Lm, labrum; Mth, mouth; Opl, operculum; Pl, pleurites; Tr, trochanter; vO, ventral sense organ; I–XIII, body segments beginning with cheliceral segment.
On the opisthosoma the closely overlapping appendages (fig. 5 B, ApdsVIII–XIII) are set in a depression of the undersurface, which is surrounded laterally and posteriorly by a broad, flat doublure with a hard, polished surface. The appendages of the first pair are united to form an operculum (Opl) covering the other five, which bear leaflike gills on their posterior surfaces. On the upper (posterior) side of the operculum are the openings of the genital ducts, situated in each sex on a pair of small papillae (fig. 12 B, gp). At the base of the tail spine is the anus (fig. 5 B, An). The appendages will be more fully described in a later section.
The prosoma and the opisthosoma are connected by a large median dorsal muscle, the arthrotergal muscle of Benham (1885), that crosses the hinge line between the two parts of the body and serves to flex the opisthosoma on the prosoma. In the ventral part of the body, long muscles arising in the prosoma are distributed by branches to the ventral wall of the opisthosoma and are evidently levators of the opisthosoma; a few ventral fibers from the prosoma, however, go to anterior dorsal apodemes of the opisthosoma and are therefore flexors, or depressors, of the opisthosoma.
The presence of seven pairs of appendages on the prosoma of Limulus should indicate that the xiphosurid prosoma contains at least seven segments. Studies on development show that all the appendages are postoral in the embryo (fig. 8 A), the definitive preoral position of the chelicerae and the first two pairs of legs being a secondary result of the posterior displacement of the mouth, by which the ventral parts of the anterior segments in the adult appear to be lapped forward around the sides of the mouth and the labrum (fig. 7 F). Limulus differs from a trilobite in having seven instead of four postoral segments in the prosoma. In appearance, however, there are only six pairs of appendages, since the chilaria have lost all semblance to legs, and in this respect the Xiphosurida resemble the Arachnida, in which the seventh segment is reduced or suppressed, and there are only six pairs of prosomatic limbs.
The area of the prosoma formed by the postoral segments cannot account for the whole of the prosomatic part of the animal, since there is a large preoral region in front of the cheliceral segment. Iwanoff (1933) has shown in a figure of a Limulus embryo (fig. 8 A) the presence of a procephalic lobe (Prc), which is a prominent anterior part of all arthropod embryos, and which appears to correspond with the acronal lobe of a young trilobite (fig. 3 A, Acr). Iwanoff, however, does not seem to attribute much of the adult structure of Limulus to this lobe, but a comparison of a larval stage of Limulus (fig. 8 B) with a trilobite would suggest that, as in the trilobites, the procephalic lobe has expanded laterally and posteriorly around the postoral segments to form the lateral parts of the prosomatic carapace, on which the compound eyes are developed. Otherwise, it is difficult to account for the likeness of the prosomatic shield of Limulus to the dorsal surface of the head of a trilobite. The forward radiation of the anterior postoral segments around the sides of the mouth indicated by Iwanoff (1933, fig. 62) probably applies only to the ventral surface of the animal. A division of the procephalic area into an ocular and an antennal segment has not been observed in the xiphosurid.
Fig. 7. Xiphosurida. Limulus polyphemus L.
A, section of prosoma behind bases of third legs. B, base of tail spine, with muscles. C, posterior part of prosomatic carapace, turned upward and separated along hinge line from anterior part of opisthosoma, showing division of eighth tergum (VIIIT) between the two parts of the body. D, inner surface of opisthosomatic carapace, and posterior part of prosomatic carapace, joined along hinge line (h). E, prosomatic endosternum of specimen 47 mm. long. F, ventral surface of prosoma with prosomatic appendages removed, showing position of their bases relative to the mouth.
1Ap, 2Ap, first and second tergal apodemes; Cp, carapace; E, compound eye; Endst, endosternum; h, dorsal hinge between prosoma and opisthosoma; 1p, 2p, external pits of first and second tergal apodemes; prsCp, prosomatic carapace; T, tergum; V, venter; z, line of removal of ventral wall of opisthosoma. Other lettering as on figure 6.
Segmentation of the opisthosoma in the adult of Limulus is indicated not only by the presence of the appendages, but also by the double series of pits on the dorsal surface (fig. 5 A). The pits are the points of ingrowth of apodemal processes on the inner surface of the back (fig. 7 D), and, as shown by Benham (1885), the muscle relation to the apodemes leaves no doubt that the apodemes arise on the primitive intersegmental lines. The first pair of the opisthosomatic apodemes (2Ap), however, lies well behind the hinge (h) between the two parts of the body, showing that the hinge is not a true intersegmental line. Moreover, there is an anteriormost pair of larger apodemes on the prosoma close to the posterior margin of the carapace (C, D, 1Ap). Therefore, the space between these prosomatic apodemes and the first pair on the opisthosoma must represent the dorsum of a segment that has been partitioned between the prosoma and the opisthosoma, and which contains the intersomatic hinge. There is nothing unusual in this condition; it occurs in other arthropods and is merely an anatomical adjustment to the necessary mechanism of movement of one part of the body on another. Since the longitudinal muscles are primarily intrasegmental, the opisthosoma of the xiphosurid can become movable on the prosoma by its own muscles only by having the anterior part of its first tergum fused with the prosomatic carapace and a line of flexibility developed across its middle.
The tergal area of the eighth body segment (fig. 7 C, VIIIT) is discernible as a small arc on the posterior border of the prosoma marked by the pits (1p) of the first dorsal apodemes, and as a wider transverse area on the opisthosoma before the second apodemal pits (2p), ending laterally in large, free, pointed lobes. The limits of the following segments can be judged only by the apodemal pits and their apparent relation to the marginal spines. The posterior part of the opisthosoma of Limulus must include a segment corresponding with the telson of other arthropods, which contains the anus. The anus of Limulus lies ventrally just before the base of the tail spine (fig. 7 D, An). It is questionable, therefore, if the spine itself represents the telson; it appears rather to be an appendage of the telson. The spine is not present in the embryo (fig. 8 A), but grows out from the end of the body during larval development (C, D). In the adult, the tail spine is an independently movable organ provided with a strong musculature (fig. 7 B) consisting of six long dorsal muscles and two large ventral muscles. The fibers of the median pair of dorsal muscles are distributed along the dorsal apodemes of the opisthosoma, those of the two lateral pairs arise on the opisthosomatic carapace; the ventral muscles divide each into a dorsal branch and a ventral branch. The origins of the spine muscles are thus far in front of the area of a presumed apical segment; their insertions, moreover, are not on the spine itself, but on a tough integument that connects the spine with the body. The spine is hollow to its tip, lined with an epithelium traversed by nerves and blood vessels.
In the decapod crustaceans and in the pterygote insects there is a more or less elaborately developed endoskeleton consisting of arms, ridges, or plates that grow in from the cuticle of the body wall and serve to strengthen the exoskeleton or to give attachment to muscles. The principal structures of this kind in Limulus are the dorsal apodemes of the carapace and the tendons of muscles. In the center of the prosoma, however, is a horizontal plate suspended by muscles from the carapace, lying between the alimentary canal and the ventral nerve cord, which, though it is not a skeletal derivative, takes the place of a ventral endoskeleton, since all the ventral prosomatic muscles are attached on it. The plate, therefore, is generally termed the endosternum; Lankester (1884, 1885) and Benham (1885) call it the “entochondrite,” or “plastron.” It differs somewhat in shape and consistency with the age of the animal. In a small specimen two inches long the endosternum is a thick membrane. In shape it is an oblong rectangle (fig. 7 E) with the anterior corners produced into a pair of spatulate anterior lobes, the posterior angles drawn out into broad posterolateral lobes; laterally on the upper surface is a pair of soft, triangular dorsal lobes, and from the posterior margin a taillike extension gives attachment to numerous muscle fibers. From the anterior end on each side two long, slender suspensory arms diverge outward and upward and are attached by muscles on the carapace. Numerous muscles depart from the margins of the plate and its lobes, and others arise directly on the dorsal and ventral surfaces. The endosternal muscles have been fully described and illustrated by Benham (1885). According to Lankester (1884), the endosternum, or "entochondrite,” of the prosoma of an adult Limulus has a texture resembling that of hyaline vertebrate cartilage, though it lacks the essential constituents of cartilage. A similar endosternal plate is present in most Arachnida, and it is probable that the ligament connecting the ventral adductor muscles of the gnathal appendages in lower Crustacea and in Diplopoda is a structure of the same nature.
In its microscopic structure, Lankester (1884) says, the adult prosomatic endosternum “is a firm homogenous, or sparsely fibrillated matrix in which are imbedded nucleated cells generally arranged in rows of three, six, or even eight parallel with the adjacent lines of fibrillation.” In a larval specimen 2 cm. in length, the endosternum is a thin membrane with a faintly fibrillated structure. Where muscles are attached on it, the fibrillae become more distinct and appear to run out directly into the fibrillae of the striated parts of the muscle fibers. The fibrillar continuity is plainly to be seen in the suspensory arms. In a specimen 5 mm. in length, the “plate” is a delicate membrane resting close upon the nerve mass beneath it, but it is not essentially different from that of older specimens. Where muscles are attached, the striated fibers break up into bundles of fine fibrillae that are soon lost in the tissue of the endosternal membrane. The endosternum is a nonchitinous tissue.
In the opisthosoma of Limulus the lateral systems of ventral muscles are segmentally connected by transverse ligamentlike bands, which are termed the “mesosomatic entochondrites” by Benham (1885) because they appear to be a tissue of the same kind as that of the prosomatic endosternum. These connectives, however, lie below the nerve cord, though they are free from the epidermis of the body wall beneath them.
The origin and nature of endosternal structures of arthropods have not been satisfactorily explained. Lankester (1885) suggests that the prosomatic endosternum of Limulus is derived from the subepidermal connective tissue of the sternal surface, but he does not explain how it brings with it the muscles, which presumably at first were attached on the cuticle of the body wall, and, to account for the definitive supraneural position of the plate, he has to assume that the endosternum of the prosoma was formed when the nerve cords had a lateral position. Schimkewitsch (1895) has described the endosternum of Arachnida as a transformed muscle tissue. It may be noted that in some insects there is in the abdomen a series of transverse muscles over the nerve cord.
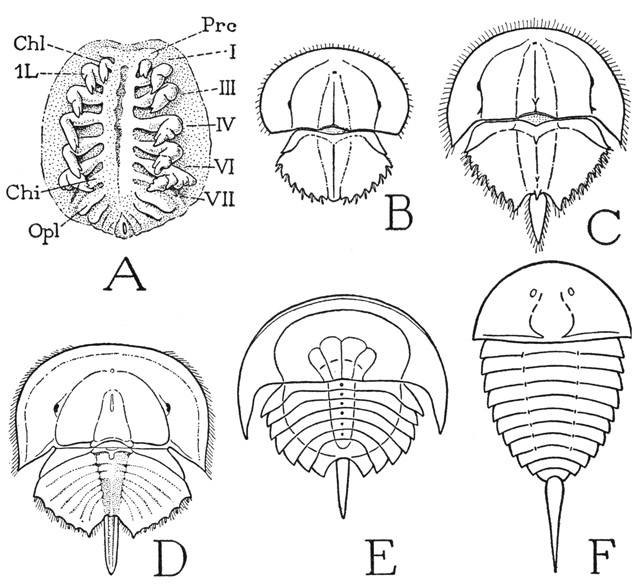
Fig. 8. Xiphosurida. Embryonic and larval stages and early fossil xiphosurids.
A, Tachypleus gigas (Müller) (Limulus moluccanus Latr.), embryo with nine pairs of appendages (from Iwanoff, 1933). B, Limulus polyphemus L., larva 4 mm. long. C, same, larva 5 mm. long. D, same as A, second larva just before moulting, opisthosomatic segments indicated by mesoderm bands (from Iwanoff, 1933). E, Prestwichianella rotundata, a Carboniferous fossil xiphosurid (from Störmer, 1944, after Woodward). F, Aglaspis aetoni, a Cambrian fossil xiphosurid (from Störmer, 1944, after Raasch).
Chi, chilarium; Chl, chelicera; Opl, operculum; Prc, cephalic lobe; I–VII, prosomatic somites.
The Eyes
The functional eyes of Limulus include the lateral compound eyes (fig. 5 A, E), and the small dorsal ocelli (dO). The ventral organ lying in front of the chelicerae (fig. 6 A, vO) has been regarded by some investigators as a pair of degenerate ventral ocelli and by others as an olfactory organ.
The Compound Eyes— Each compound eye is covered by a thick, convex cornea having an entirely smooth surface, but on looking into it one sees in its deeper part a regular, honeycomblike pattern of minute, six-sided facets. This appearance is not due to any division of the cornea; an examination of the inner surface shows that the latter is projected into numerous small, peglike processes so arranged that the spaces between them have a hexagonal shape. Each corneal process (fig. 9 A, Ln) serves as a lens for a group of sensory cells (Om) lying beneath it, which is ensheathed in long cells of the epidermis (B). Each group of cells associated with a lens of the common cornea, therefore, is a light-receptive unit of the eye corresponding with an ommatidium of the compound eye of higher arthropods. The sensory cells constitute the retina, or retinula, of the ommatidium, and are closely arranged in a radial manner around a central axis (C, rSCl), so that, as Demoll (1914) says, the ommatidial retina resembles a peeled orange. The number of radial cells in a single ommatidium varies from 10 to 15; their inner ends are separated by an axial space, in which, according to Demoll, is a long, slender process (axp) from an excentric retinal cell (B, ecSCl) lying outside the bases of the other cells. The narrow inner margins of the radial cells and the axial process of the excentric cell are composed of a relatively clear cytoplasm with fine striations at the surface (B, C, sb). In the compound eyes of most arthropods the striated borders of the convergent retinal cells unite to form an axial, rodlike structure called a rhabdom, and are therefore termed rhabdomeres. The function of the rhabdom or the rhabdomeres is the diffraction of light from the lens into the light-sensitive parts of the retinal cells. Proximally the retinal cells are continued into nerve processes (B, Nv) that go to the brain. The corneagenous epidermal cells of the eye (B, CgCls) that surround the lens and ensheath the retina are shown by Demoll to converge between the lens and the retina, but they do not form here any specific dioptric body such as the crystalline cone of most compound eyes. The lateral eyes of Limulus are thus seen to be true compound eyes, but to have a simpler structure than those of higher arthropods.
The Dorsal Eyes— The small, median dorsal eyes of Limulus, as described by Demoll (1914), are typical arthropod ocelli. The cornea of each of these eyes (fig. 9 D, Cor) is produced inwardly as a large spherical lens (Ln), which is enclosed in a layer of corneagenous cells (CgCls) continuous at the periphery of the eye with the surrounding epidermis (Epd). Beneath the corneagenous layer is the relatively thick retina, composed of the sensory cells (SCls), scattered interstitial cells, and a binding network of connecting tissue that forms also a limiting membrane around the base of the eye, penetrated by the nerve processes (Nv) of the sensory cells. At one side of the eye is a mass of undifferentiated cells (e) which Demoll regards as a degenerate eye, and he draws the inference that Limulus once had a group of four dorsal ocelli.
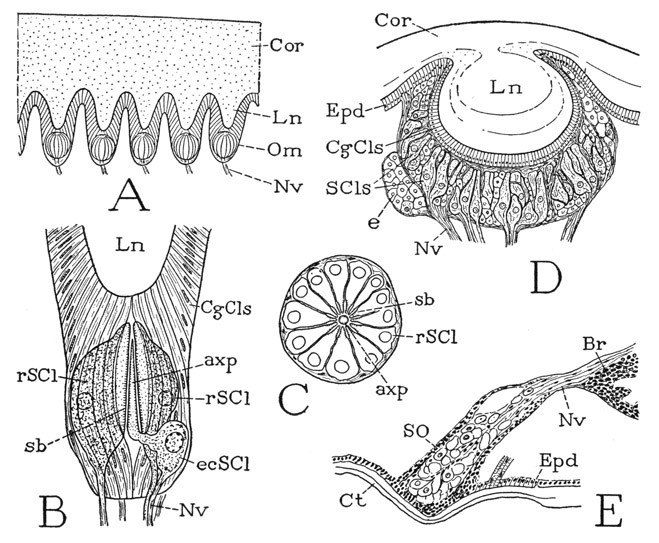
Fig. 9. Xiphosurida. Eyes of Limulus.
A, diagrammatic section of the cornea and five ommatidia of compound eye (from Lankester and Bourne, 1883). B, somewhat diagrammatic axial section of an ommatidium of compound eye (from Demoll, 1914). C, cross section of an ommatidium of compound eye (outline from Demoll, 1914). D, axial section of a dorsal ocellus (from Demoll, 1914). E, sagittal section of a “ventral eye” and nerve from brain (from Demoll, 1914).
axp, axial process of eccentric sensory cell (ecSCl); Br, brain; CgCls, corneagenous cells; Cor, cornea; Ct, cuticle; e, mass of undifferentiated cells; ecSCl, eccentric sensory cell; Epd, epidermis; Ln, lens; Nv, nerve; Om, ommatidium; rSCl, radial sensory cell; sb, striated border; SCls, sensory cells; SO, ventral sense organ.
The “Ventral Eyes”— The supposed ventral eyes of Limulus (fig. 6 A, vO) are described by Demoll (1914) as consisting of two masses of irregularly arranged cells (fig. 9 E) abutting on the epidermis beneath convexities of the cuticle. Each organ consists for the most part of large cells associated in groups, with connective tissue between them. The large cells are produced into nerve fibers that go to the brain (Br), and where two of the cells adjoin each other their opposed surfaces, Demoll says, have rhabdomere borders, though they do not form rhabdoms. These cellular bodies certainly must be, or have been, sensory organs of some kind, and probably the idea that they are degenerate eyes is as good as any other; their structure does not suggest an olfactory function.
The Appendages
We may now give special attention to the appendages, including the seven pairs on the prosoma and the six on the episthosoma. A full account of the embryonic development of the appendages in Limulus moluccanus is given by Iwanoff (1933), who shows that all the appendicular organs, regardless of their differences in the adult stage, begin in the same way, as a pair of folds of the ventral body wall.
The Chelicerae— Since the Xiphosurida lack antennae, the first of the prosomatic appendages are the chelicerae (fig. 5 B, Chl), which, as already noted, lie directly in front of the mouth in the adult state, but take this position secondarily from a primary postoral position in the embryo (fig. 8 A). The chelicerae presumably represent the first postoral appendages of the trilobites. In the adult of Limulus the chelicerae are articulated on a small plate (fig. 6 B, Epst) that evidently is the epistome, since it supports the labrum (Lm) and is produced on each side into a slender arm curving forward along the mesal border of the coxa (Cx) of the first leg. Each chelicera is three-segmented (fig. 10 D), but the terminal segment is a slender claw, or movable finger, opposed to a similar fixed process of the second segment, so that the two together form a pair of pincers. According to Benham (1885), the chelicerae are movable by muscles inserted on their bases that arise on both the carapace and the endosternum.
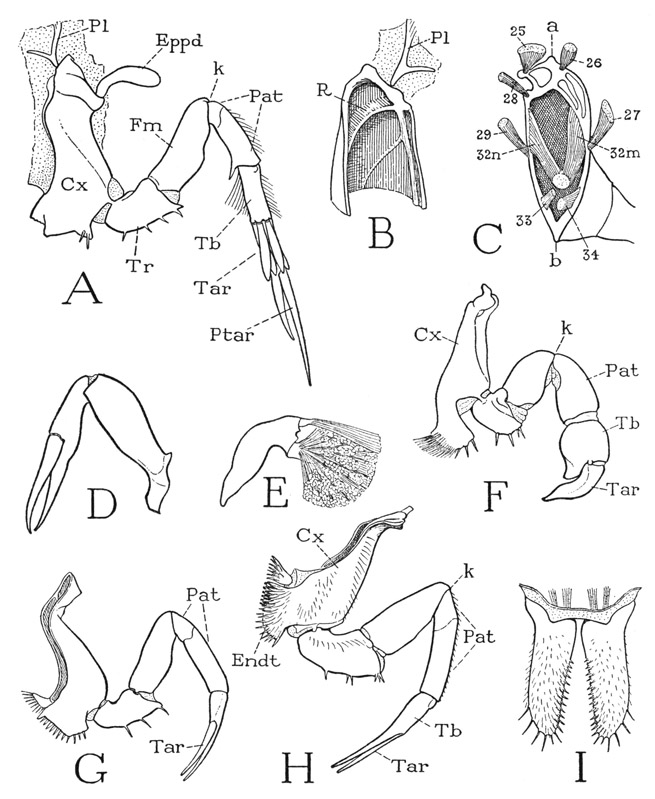
The Legs— The five pairs of legs (fig. 5 B, L) resemble the chelicerae in that their distal segments form pincers, but they have a greater number of segments, and in the adult male the chelae of the first legs assume an altered form (fig. 10 F). The basal segments, or coxae, of the legs are greatly elongate dorsoventrally and are attached on the membranous lateral walls of the deep median part of the prosoma beneath the lateral extensions of the carapace (fig. 7 A, Cx); their only firm articulations are with the small Y-shaped pleural sclerites in the membranous inner part of the doublure (fig. 6 C, Pl). The lower ends of the coxae of the first four pairs of legs are produced into large spine-covered mesal lobes, or endites (fig. 10 H, Endt), the spines of which curve forward toward the mouth (fig. 6 A). Above the endites of the second, third, and fourth legs is a small accessory endite arising from the membrane at the base of the coxa (fig. 10 G, H).
Each leg is movable anteriorly and posteriorly on the long base of its coxa (fig. 10 C, a–b), but also has a transverse movement because of the flexible, membranous nature of the body wall on which it is attached. As shown by Benham (1885), nine muscles are inserted on the basal rim of the coxa (fig. 10 C), five of which (25, 26, 27, 28, 29) are dorsal muscles arising on the carapace, and four (32m, 32n, 33, 34) are ventral muscles with their origins on the endosternum. Two of the ventral muscles (32m, 32n) branch from a common origin. The anterior and posterior dorsal muscles evidently are promoters and remoters, that is, they rotate the coxa on its dorsoventral axis (a–b); the ventral muscles must have an adductor function because of the dorsal suspension of the coxa on the pleural sclerite. Since there is no evident muscular mechanism to oppose the ventral adductors, abduction of the limb apparently results from the elasticity of its basal connections.
The legs of the first four pairs have each only six segments; the somewhat longer last legs, however, have seven segments, and therefore represent a more nearly complete appendage, for which reason they should be studied first. The coxa of a fifth leg (fig. 10 A, Cx) resembles the coxae of the other legs except for the absence of spines on the endite lobe. A short section of its upper end is set off by a groove marking the base of a strong internal ridge (B, R), and bears a spatulate epipodite (A, Eppd). The next segment is the trochanter (Tr), which turns on the coxa in a vertical plane, and supports the longer third segment, which is the femur (Fm). At the end of the femur is the “knee” bend of the leg (k), beyond which are four segments. The segment adjoining the femur very evidently corresponds with the segment of an arachnid leg called the patella (fig. 19 A, Pat), and the following segments of the Limulus leg should then be the tibia (Tb), the tarsus (Tar), and the pretarsus (Ptar). A different idea concerning the identity of these segments, however, has been given by Hansen (1930), who regards the faint circular groove near the base of the segment beyond the knee as the division between patella and tibia, so that he has to invent the name “cotibia” for the next segment. The musculature of the leg, as will presently be seen, gives no support to this interpretation. The basal groove is present also on the preceding three pairs of legs (G, H), but not on the first legs (F). The segmentation of the hind leg of Limulus corresponds with the apparent segmentation of a trilobite leg (fig. 4 B), except for the lack of a second trochanter, or prefemur. The patellotibial joint has a characteristic structure (fig. 11 C), there being two dorsal lobes on the tibia, between which a median, tonguelike process curves down from the end of the patella. The end of the tibia of the last leg (fig. 10 A) bears four bladelike appendages that conceal the cylindrical tarsus. The pretarsus (Ptar) is a long, tapering, spinelike segment, which, with a shorter process projecting from the end of the tarsus, forms a slender chela.
In studying the other legs, the question arises as to what segment has been eliminated to leave only six segments; the answer is furnished by the musculature. The intrinsic musculature of the hind leg is sufficiently shown at A of figure 11, though some of the muscles of the inner, or posterior, side of the leg are not seen in the anterior view. Since there is no question as to the identity of the first three segments in all the legs, we may note first the muscles that move the part of the leg beyond the knee. In the ventral membrane of the knee joint is a small V-shaped sclerite. The arms of the V give attachment to a pair of broad muscles (8) arising dorsally in the femur; the apex is produced into a long, thick, rodlike apodeme, or tendon (t), that extends through the femur and gives attachment to a thick conical muscle (10) in the trochanter. These three muscles are present in all the legs and serve to flex the distal part of the limb on the femur. The patella contains six muscles inserted on the base of the tibia; two are large dorsal muscles (12), two are fan-shaped lateral muscles (14), and two are long slender ventral muscles (16). Each ventral muscle has two branches, one branch (16a) arising in the distal end of the femur, the other (not seen in the figure) in the base of the patella. The tarsal muscles in the tibia are a dorsal levator of the tarsus (18) and a two-branched ventral depressor (19). The pretarsus has a simple levator (20) and depressor (21) arising in the tarsus.
Fig. 10. Xiphosurida. Limulus polyphemus L. The prosomatic appendages.
A, left last leg, anterior. B, inner surface of anterior wall of upper end of coxa. C, base of leg with attached muscles (from Benham, 1885). D, left chelicera, lateral. E, movable finger and muscles of chela of first leg of adult male. F, first left leg (pedipalp) of adult male, anterior. G, right third leg, posterior. H, left fourth leg, anterior. I, chilaria, posterior.
a, dorsal (pleural) articulation of coxa; b, ventral articulation of coxa; Cx, coxa; Endt, coxal endite; Eppd, epipodite; Fm, femur; k, knee bend of leg; Pat, patella; Pl, pleurite; Ptar, pretarsus; R, ridge of coxal wall; Tar, tarsus; Tb, tibia; Tr, trochanter.
If now we compare the musculature of one of the other legs with that of a hind leg, it is found that the first segment beyond the knee has the same flexor mechanism as this segment in the hind leg, and that it contains the same muscles (fig. 11 B). This segment, therefore, is the patella in all the legs (fig. 10 A, F, G, H, Pat). Between it and the next segment (fig. 11 B, Tb) there is no suggestion of a reduced or eliminated segment, and the nature of the musculature from the patella leaves no doubt that this segment is the tibia. Moreover, the structure of the joint between these two segments is exactly that of the patellotibial joint of the hind leg (C). Finally, the musculature in the tibia of the anterior legs is that within the tibia of the hind leg inserted on the tarsus. We must conclude, therefore, that in all the legs but the last, the terminal segment forming the movable finger of the chela is the tarsus (fig. 10 G, H, Tar), and that the opposed finger is a process of the tibia. Hansen (1930) made the same deduction without a study of the musculature. The chela of the anterior leg becomes much modified in the adult male (fig. 10 F). The tibia, or “hand” of the chela (Tb), is short but greatly swollen, and the long clawlike tarsus, or movable finger (Tar), is thick and bent, while the immovable process of the tibia is reduced to a short thumb. The globular tibia contains a small dorsal muscle of the tarsal claw (E) and two great masses of closing fibers inserted on a thick knob of the tarsal base. This seemingly deformed chela of the male, therefore, evidently has great strength.
The Chilaria— The appendages of the seventh postoral body segment, known as the chilaria, lie close to the posterior margin of the venter of the prosoma (fig. 6 A, Chi), between and behind the coxal endites of the last pair of legs. Each chilarium (fig. 10 I) is a small, simple, elongate, flattened lobe, armed with spines on its distal end and along its mesal margin. The two appendages arise close together and project downward, so that they close the posterior end of the space between the leg bases leading forward to the mouth (fig. 6 A). From their position the chilaria have acquired their name, cheilarion being Greek for a “little lip.” According to Iwanoff (1933), the chilaria correspond with only the coxal endites of the preceding appendages. They have a very weak basal musculature.
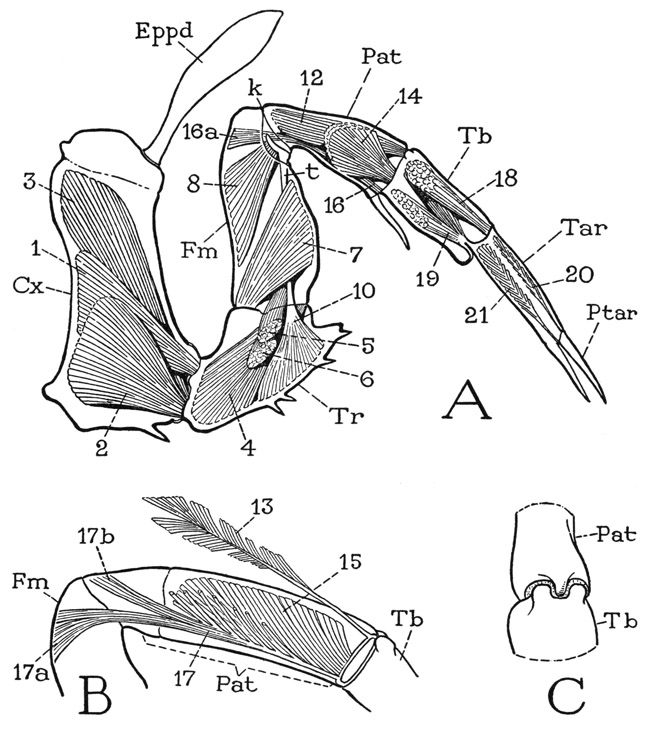
Fig. 11. Xiphosurida. Limulus polyphemus L. Structure and musculature of a leg.
A, left last leg and intrinsic musculature, anterior. B, patellar segment, and muscles of posterior half. C, patellotibial joint, dorsal, structure characteristic of this joint in all the legs.
Lettering as on figure 10; muscles explained in text.
The Opisthosomatic Appendages— The six appendages of the opisthosoma are broad, flat, closely appressed, horizontal plates lying in the ventral concavity of the opisthosoma (fig. 5 B, ApdsVIII–XIII), where they underlap from before backward. The first plate is a large operculum (Opl) mostly covering the others. Each plate is provided with a pair of promotor muscles, and a pair of remotor muscles arising on the dorsum of the body wall (fig. 12 A, B), both being inserted within the cavity of the appendage, the smaller promotor (pmcl) on the anterior lamella, the larger remotor (rmcl) on the posterior lamella. There is little reason to suppose that these opisthosomatic appendages of Limulus are not true segmental limbs, but it is difficult to identify their parts with those of a prosomatic leg.
The operculum hangs from the membranous ventral wall of the body between the prosoma and the opisthosoma, but, as we have seen, the tergum of the first opisthosomatic segment is partitioned between the opisthosoma and the prosoma. Accordingly, the promotor muscles of the operculum arise on the posterior apodemes of the prosomatic carapace, and the remotors on the anterior margin of the opisthosoma. The operculum differs from the following appendages in that its lateral halves are more closely united along the mid-line. On the anterior surface (fig. 12 A) two median series of sclerites appear to represent the shafts of the component appendages, and the broad lateral expansions can be interpreted as basal exites, probably coxal epipodites. A transverse line of flexibility (1–1) through the distal part of the operculum cuts off the apical parts of the exites as a pair of free lateral lobes and separates the two distal segments from the long basal segments of the median shafts, the apical segments of which form a pair of small, free median lobes. The posterior surface of the operculum (B), however, gives little evidence of limb structure, except for the apical lobes. At about the middle of the basal part are two small genital papillae (gp), present alike in both sexes, which contain the outlets of the genital ducts (Dct). Since the operculum of Limulus pertains to the eighth postoral body segment, it is of interest to note that the genital opening in Arachnida also is on the eighth segment.
In the next five appendages of the opisthosoma (fig. 12 C, D) the broad lateral parts are separated by a wide, median membranous area, beyond which the component appendages are represented by a pair of three-segmented median lobes, with a slender tongue of the basal membrane projecting between them. On the posterior surfaces of the appendages, turned upward, are the gills (D, Brn). The gills consist of two great lateral masses of thin, closely superposed branchial lamellae, at least 80 of them in a single gill mass of an adult specimen. Each gill is an extremely thin semilunar plate with a rigidly thickened margin fringed with delicate setae. The lamellae are attached transversely by their bases on the supporting appendage and slope distally on the latter.
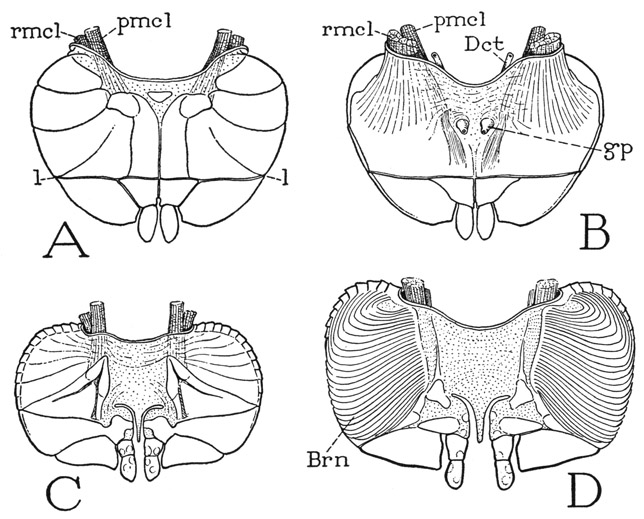
Fig. 12. Xiphosurida. Limulus polyphemus L. Opisthosomatic appendages.
A, operculum, anterior. B, same, posterior. C, united second pair of appendages, anterior. D, same, more enlarged, posterior.
Brn, gills (branchiae); Dct, genital duct; gp, genital papilla, with genital orifice; l, fine of flexion; pmcl, promotor muscle; rmcl, remotor muscle.
Relationships of the Xiphosurida
A comparison of Limulus with a trilobite can hardly fail to give the impression that the two are related animals, though the principal likeness is in the form and structure of the prosoma, with its wide lateral parts inflected ventrally before the mouth and around the sides of the appendages. On the other hand, in the lack of antennae, the absence of segmentation in the opisthosoma, and the differentiation of the appendages, Limulus is far more specialized than a trilobite. The xiphosurids, however, have an ancient lineage; Limulus itself in almost its present form goes back to Jurassic times in the Mesozoic Era. Its ancestors in the Carboniferous have distinctly segmented abdomens (fig. 8 E), as do still earlier Paleozoic representatives that lived in the Cambrian (F). The two-part bodies and the tail spine of these ancient forms leave no doubt that they are xiphosurids. The union of segments in the opisthosoma, therefore, is only a specialization of the comparatively recent Limulus.
Associated in the Cambrian with the early xiphosurids and the trilobites were many other arthropods whose known remains show such a mixture of characters that paleontologists cannot agree among themselves just what they are, whether trilobites, xiphosurids, eurypterids, or crustaceans, which fact is probably good evidence that the animals themselves represent early intermediate forms between these several groups. Limulus, however, has survived all its ancient relatives and still flourishes in modern times, so it is not surprising that it has become the most specialized member of its tribe. Störmer (1944) has interestingly described and pictorially illustrated the evolutionary history of the xiphosurids, showing examples of the different forms found at successive paleontological levels. It is not necessary to suppose, however, that the Xiphosurida have been derived directly from trilobites; it is a safer assumption that the two groups of animals had a common ancestry.