THE CRUSTACEA
THE Crustacea introduce us to the arthropod group known as the Mandibulata, which includes also the chilopods, the diplopods, the pauropods, the symphylans, and the hexapods, or insects. The Mandibulata are so named from the fact that their principal organs for biting and chewing are a pair of jaws, the mandibles, fashioned from a pair of segmental appendages that correspond with the pedipalps of the Chelicerata. In the Crustacea the mandibles are preceded by two pairs of antennae. The second antennae evidently are represented by the chelicerae in the Chelicerata, but they are absent in mandibulate forms other than the Crustacea, except for transient embryonic vestiges. The first antennae, or antennules, however, are characteristic head appendages of all the mandibulate arthropods and are present in the trilobites. Since the antennules do not have the structure of the following appendages, it is probable that they represent very primitive head appendages of the ancestral arthropods, which have been lost only in the chelicerates and in a few forms here and there among the mandibulates. The second antennae, on the other hand, show by their structure that they belong to the series of body limbs.
The postantennular appendages of the Crustacea have typically a biramous structure, owing to the frequent presence of a lateral branch on the second segment from the base of the limb. The branch is termed the exopodite, and the main shaft of the limb beyond it the endopodite. The biramous limb is probably a primitive crustacean character, but by a suppression of the exopodite the appendage in many cases reverts to a simple uniramous form. Since the Crustacea are generally regarded as the most generalized of the mandibulate arthropods, the biramous limb has been thought to be the primitive form of the arthropod appendages. Occasional branched limb structures in other mandibulates, therefore, have been interpreted as retentions of, or reversions to, the ancestral structure. Such interpretations, however, are in no case necessary, and are not supported by specific evidence; the exopodite branch of the limb is a crustacean specialty. Besides the exopodite, there may be an epipodite borne on the outer face of the coxopodite, and median lobes, or endites, on the coxopodite and basipodite. A determination of the homologies of the limb segments and their accessories is one of the interesting and often perplexing problems in carcinology.
The Crustacea include many diverse forms; taxonomically they have been divided into two major groups, the Entomostraca and the Malacostraca. The term Entomostraca, however, is just a convenient name for a large number of small crustaceans such as the branchiopods, the ostracods, the copepods, and the barnacles that may have no close relationships to one another. The Malacostraca, on the other hand, including such forms as the shrimps, crayfish, lobsters, and crabs, are a more homogeneous group, but included in it are the amphipods and the isopods, which have distinctive characters of their own. A chapter on the Crustacea in general would expand to the size of a book. Hence, we shall have to omit the entomostracans entirely and limit a discussion of the Malacostraca to three examples, Anaspides, a crayfish, and an isopod, representing the principal types of malacostracan organization.
ANASPIDES
Anaspides is a small, shrimplike crustacean known only from Tasmania, where it lives in pools of running water on Mount Wellington, mostly above 1,400 feet. A related form, Paranaspides, inhabits the Great Lake of Tasmania at a height of 3,700 feet, and another, Koonunga, has its home in Australia. A minute crustacean named Bathynella, found in springs and caves of central Europe, is usually classed with the Anaspidacea, but it has a number of quite distinctive features. The Tasmanian and Australian species are nearest related to certain fossil crustaceans from Permian and Carboniferous strata of Europe and North America, and they are the only known living representatives of this group, called the Syncarida, which includes several families comprised in the single order Anaspidacea. For earlier discussions on the syncarids, the student is referred to Smith (1909) for the structure of the Anaspidacea, living and fossil, to Calman (1896) for a demonstration of the affinities of Anaspides with the fossil syncarids, and to Manton (1930) for an account of the habits and feeding mechanisms of Anaspides and Paranaspides. Koonunga is described by Sayce (1908), Bathynella by Chappuis (1915) and by Calman (1917).
Anaspides tasmaniae Thomson (fig. 37 A) has a slender, uniformly segmented body, two pairs of long antennae, and a series of segmental limbs that are mostly locomotor in function. Ordinary specimens are about an inch and a half in length, but exceptional individuals are said to exceed two inches. While Anaspides cannot be regarded as a primitive crustacean, it is unquestionably the most generalized of modern Malacostraca, and its simplicity of structure suggests that in some respects it preserves features more primitive in form than those of the Entomostraca. Hence, though specimens may not be available for class use, a description of Anaspides will give the student an outline of the essential crustacean characters in their simplest available form.
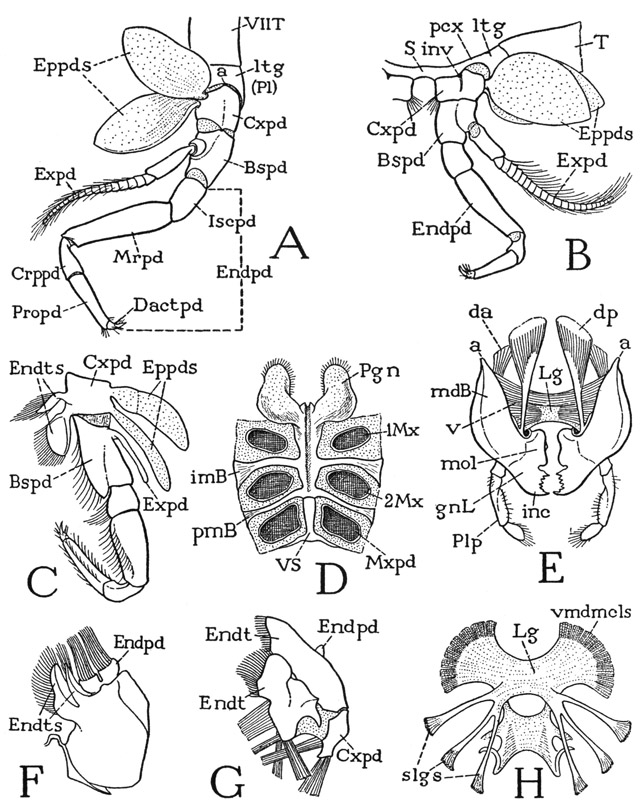
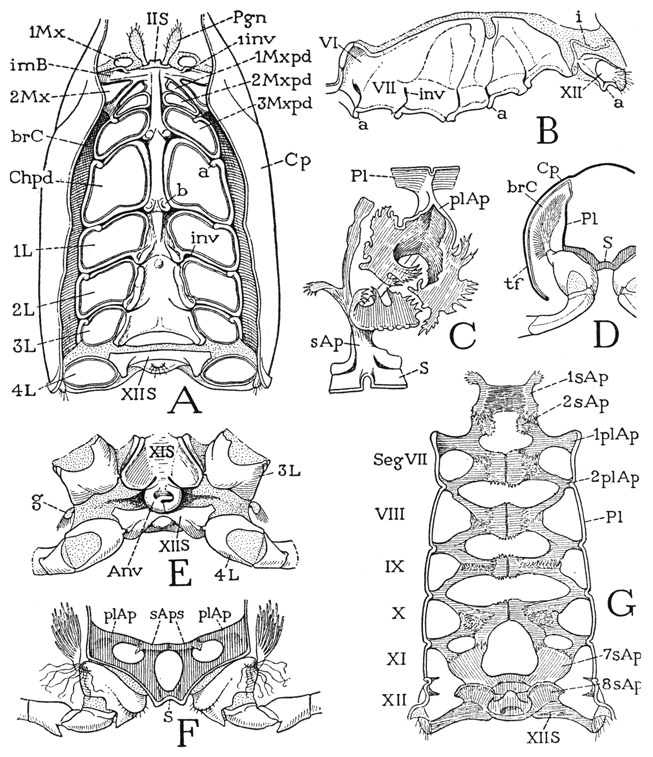
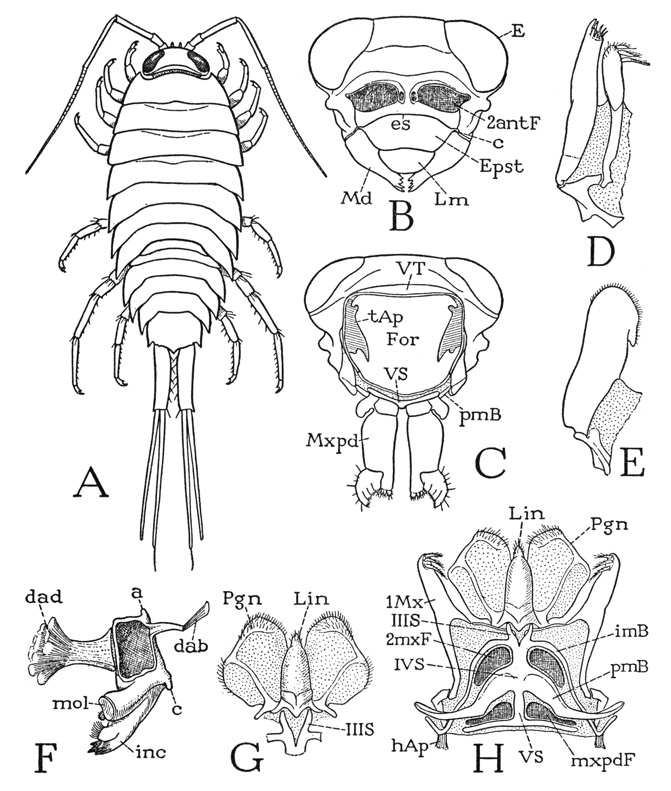
The body of Anaspides (fig. 37 A) is segmented throughout its length and bears 17 pairs of appendages, not including the two pairs of antennae. There appear to be, however, only 15 segmental divisions of the body as indicated by the number of tergal plates, but it is found that the second apparent tergum corresponds to three pairs of appendages, so that it must be a composite of three primitive segments (III + IV + V), making 17 in all (II–XVIII). The large first tergum is enumerated as belonging to segment II because in the crustacean embryo there is a preceding segment of the second antennae, corresponding with the cheliceral segment I of the Arachnida. Anterior to the antennal segment of the embryo is a primitive head lobe that bears the eyes and the first antennae. This head lobe was perhaps itself segmented at some early stage of arthropod evolution, but arthropodists do not agree as to this, or as to how many segments may be represented in the cephalic lobe of modern embryos. Merely for purposes of comparative enumeration, therefore, the crustacean segments will be numbered the same as those of the Arachnida, beginning with the segment of the second antennae as segment I. The body of Anaspides terminates posteriorly with a flat lobe, known as the telson (Tel), which contains the anus on its undersurface (fig. 39 B, An). The telson, however, has no appendages and is not regarded as a true segment, or, more properly, a somite.
The first tergal plate of the body (fig. 37 D, II) projects anteriorly in a short rostrum (R) over the eyes and the bases of the antennae. Concealed beneath the rostrum and the free edge of the tergum is a small head structure, which, when detached (B), is seen to carry the stalked eyes (E), the first and the second antennae (1Ant, 2Ant), and the labrum (Lm); it, therefore, must include the embryonic head lobe and at least a part of the second antennal segment. Since no simpler cephalic structure than this headpiece of Anaspides is known in any other adult arthropod, it may be designated a protocephalon, implying that it represents the first adult head in the evolution of the arthropods. The protocephalon recurs as a discrete head in many other crustaceans, but it is to be noted that it is principally a sensory tagma, since it does not carry the organs of feeding. In various crustaceans, however, and in all the other mandibulate arthropods, the protocephalon is united with some of the anterior body segments to form a secondary head of more complex structure that combines in one unit both the sensory and the feeding functions. Though the protocephalon is a distinct anatomical unit in the adult structure of most of the malacostracan Crustacea, and always carries the second antennae, its segmental composition is not clear, since, in the decapods at least, the dorsal muscles of the second antennae take their origins on the anterior part of the mandibular region of the carapace.
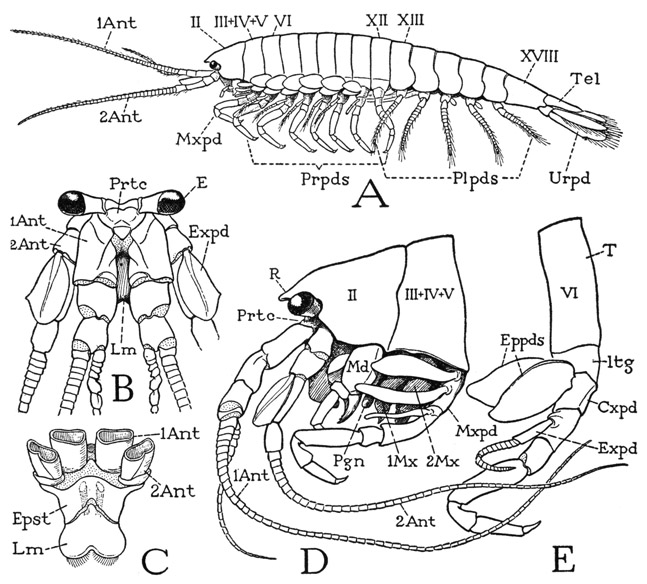
Fig. 37. Crustacea—Anaspidacea. Anaspides tasmaniae Thomson.
A, entire animal, male, with appendages of left side. B, head and its appendages, anterior. C, epistome and labrum, with bases of antennae, ventral. D, head and anterior part of body, with appendages, showing body segments of first and second maxillae and maxillipeds combined under one tergal plate (III + IV + V). E, body segment of first legs (first free segment).
For explanation of lettering see pages 190–192.
The head of Anaspides, as above noted, carries the eyes, the first antennae, and the second antennae; the stalked compound eyes (fig. 37 B, E) project laterally from the dorsal wall, the antennae arise beneath them. The first antennae, or antennules (1Ant), consist each of a thick basal stalk composed of three large segments and of a pair of slender, multiarticulate flagella, of which the outer flagellum is much longer than the inner. Though the first antennae are two-branched, the branches do not conform with those of the second antennae and the legs known as the exopodite and the endopodite, since they arise from the third and not the second segment of the appendage, and neither of them is truly segmented. The eyes and the first antennae pertain to the primary cephalic lobe of the embryo.
The second antennae (fig. 37 B, D, 2Ant) have each a basal stalk of four segments, the last of which bears a single, long, multiarticulate flagellum. From the second segment, however, arises laterally a large, flat lobe (B, Expd), which thus in position corresponds with the exopodite of a leg. The second antennae, therefore, appear to be truly biramous appendages serially homologous with the body limbs; though they are carried by the head of the adult, their rudiments in the embryo are formed behind the mouth, the appendages later becoming secondarily preoral, just as do the chelicerae in Arachnida.
The under wall of the head is formed by a wide plate beneath the bases of the antennae (fig. 37 C, Epst). This plate is the epistome of the Crustacea, and, as in the arachnids, it carries the labrum (Lm), Its basal angles are produced into narrow arms that go upward behind the antennal bases to the dorsum of the head. The labrum represents the anterior pole of the arthropod; its ventral position behind the antennae is secondary.
The trunk segments of Anaspides are not entirely uniform: there is an abrupt change in the shape of the tergal plates between segments XII and XIII (fig. 37 A). The last six segments of the body (XIII–XVIII) constitute the abdomen, or pleon, of malacostracan Crustacea; the preceding part of the body is commonly termed the cephalothorax. In Anaspides, however, the latter includes the protocephalon, the first four postcephalic segments of the body (II–V), which carry the manibles (D, Md), two pairs of maxillae (1Mx, 2Mx), and a pair of leglike maxillipeds (Mxpd), while a third region, composed of segments VI–XII (A), bears the seven pairs of walking legs, or pereiopods (Prpds), and may be termed specifically the thorax to distinguish it from the gnathal region of the feeding appendages, though generally the maxilliped segment is reckoned as a part of the thorax. The appendages of the abdomen are termed pleopods (Plpds), but the broad, leaflike appendages of the last segment are distinguished as uropods (Urpd).
The tergal plate of the dorsum of each body segment in the thoracic region comes down on the sides to the bases of the legs, but shortly above each leg base it is crossed by a faint longitudinal groove that sets off from the main plate of the back a small laterotergite (fig. 38 A, ltg), on which the coxa of the leg is weakly articulated (a). The laterotergites of Anaspides, therefore, are evidently homologous, by position at least, with the limb-supporting sclerites of other arthropods, called the pleurites, such as the small pivotal sclerites of the legs in Limulus (fig. 7 F, Pl) and the segmental components of the highly developed pleural plate of the thorax of the crayfish (fig. 43 B). The pleuron of the crustaceans thus appears to be a derivative of the dorsal skeleton.
The ventral surfaces of the first six leg-bearing segments of Anaspides are mostly membranous, but in each segment (fig. 38 B) there is a narrow, transverse sternal sclerite (S) behind the leg bases, which is continuous on each side with the laterotergite (ltg) of its segment by a postcoxal arm (pcx). Between the sternum and each coxa is a small pit (inv), which marks the root of a short internal apodeme. The sternum of the last leg-bearing segment in the male is a large plate lying before the coxae, and behind it are the paired, slitlike male genital apertures (fig. 39 D, Gprs). On the venter of the same segment in the female, between the bases of the legs, is the single aperture of the sperm receptacle. The female genital openings are on the mesal surfaces of the coxae of the fifth legs (segment X). The sterna of the maxillary segments and of the abdomen are somewhat differently developed, and will be described in their appropriate places.
The body appendages of the higher Crustacea are so diversified in form by adaptation to different functions that no general description can be made to fit them; yet it is to be presumed that they have all evolved from a common generalized type of limb. The dominance of the leg type of structure in all arthropods and the fact that the trilobite limbs are legs of uniform pattern and function suggest that in the Crustacea the typical ambulatory pereiopods have deviated least from the original limb structure. In studying the body appendages of Anaspides, therefore, we may proceed best by first understanding the structure and segmentation of the walking legs.
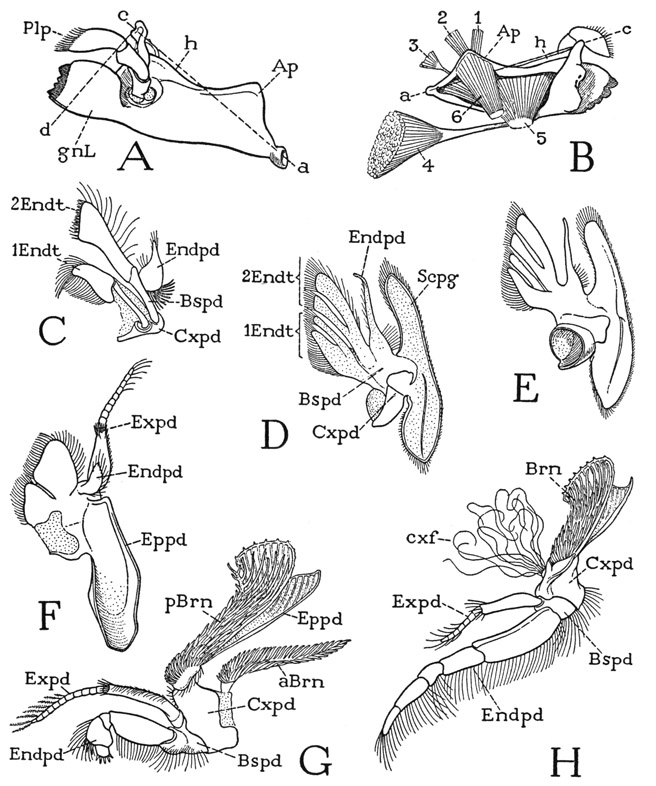
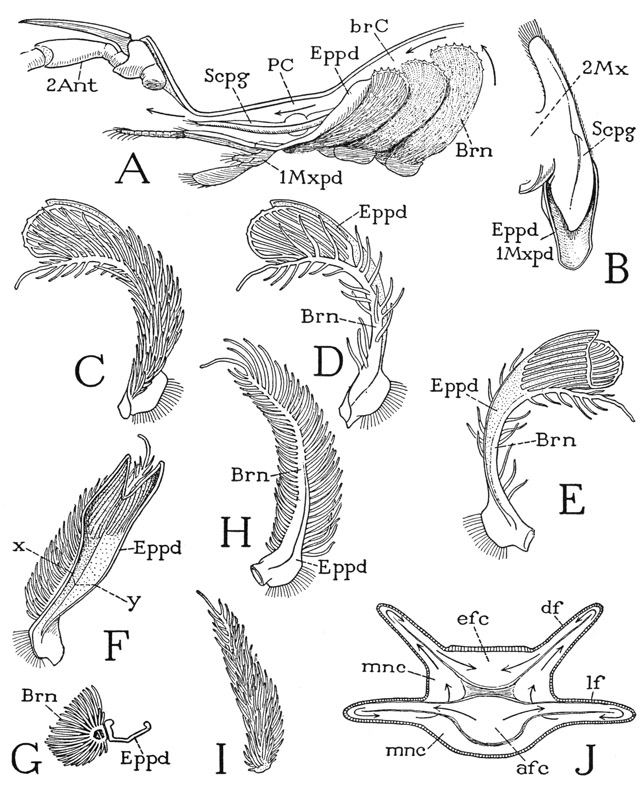
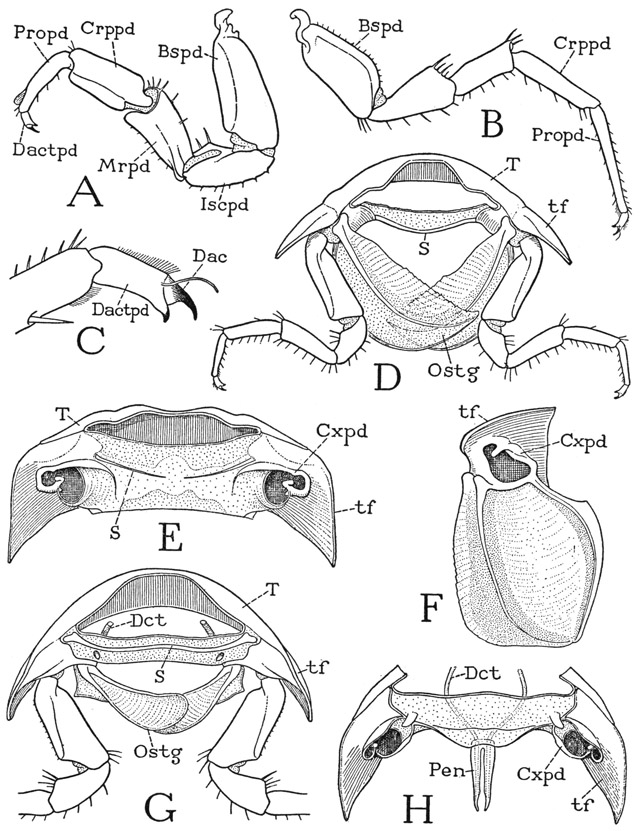
Any one of the first five pereiopods of Anaspides will serve to illustrate the typical structure of a crustacean appendage. The limb consists of a main shaft of seven segments (fig. 38 A). In the terms of carcinology, the first segment is the coxopodite (Cxpd), the second the basipodite (Bspd), the third the ischiopodite (Iscpd); then comes an elongate segment known as the meropodite (Mrpd), followed by a short carpopodite (Crppd), a slender propodite (Propd), and finally the clawlike dactylopodite (Dactpd). The knee bend of the leg is at the joint between the meropodite and the carpopodite. These two segments of the crustacean leg correspond with the femur and tibia of the arachnid leg (fig. 25 A), and consequently a patella is absent in Crustacea, as it is in the legs of all the other mandibulate arthropods. The coxopodite of Anaspides (fig. 38 A) is weakly articulated (a) on the small laterotergal plate of the dorsum (ltg), and bears on its outer surface a pair of large, flat, leaflike lobes (Eppds), which functionally are probably gills; but, in general, appendicular structures arising from the outer side of the coxopodite are termed epipodites. From the basipodite arises laterally a long slender branch of the limb (Expd), consisting of a two-segmented basal stalk and an annulated flagellum. This lateral branch of the basipodite is termed the exopodite, and the shaft of the limb beyond the basipodite is then called the endopodite (Endpd). The pereiopods of the sixth pair in the male (fig. 37 A) differ from those preceding in that the gill lobes are relatively small and the exopodite is a simple short, unjointed appendage. The last pereiopods in each sex have neither epipodites nor an exopodite.
Fig. 38. Crustacea—Anaspidacea. Anaspides tasmaniae Thomson.
A, third left pereiopod and adjoining part of tergum, lateral. B, third right pereiopod and supporting parts of body segment, posterior. C, left maxilliped, anterior. D, ventral surfaces of maxillary and maxilliped segments, with paragnaths, showing intersegmental sternal brachia. E, mandibles and their musculature, posterior. F, left second maxilla, posterior. G, left first maxilla, posterior. H, intergnathal ligament and suspensory branches (flattened under cover glass).
For explanation of lettering see pages 190–192.
Though each leg of Anaspides is clearly divided into only seven interarticulated segments, some carcinologists contend that the leg really contains eight segments, or even nine. One of these supposed segments is seen in the demarked area of the basipodite in Anaspides that bears the exopodite (fig. 38 A); but there is nothing to indicate that this part was ever an independently movable section of the leg. The idea that the primitive arthropod limb included a “subcoxal,” or “precoxal,” segment has been much exploited, and in Anaspides the small laterotergal plate of the dorsum, on which the coxa is articulated, has been interpreted by Hansen (1925) as a remnant of this hypothetical basal segment. It is on the coxa, however, as in the arthropods generally, that the body muscles of the limb are inserted, indicating that the coxa is the true base of the appendage. In Anaspides the dorsal muscles of the legs pass over the laterotergites to insert on the coxae, and there is nothing to indicate that the laterotergites are anything else than lateral subdivisions of the tergum, representing the pleural sclerites more elaborately developed in some other forms.
The maxillipeds of Anaspides (fig. 38 C) resemble the legs and have the same number of segments. The coxopodite of each maxilliped also has two epipodites (Eppds), but they are much smaller than those of the legs, and the exopodite (Expd) is reduced to a small, simple, slender appendage of the basipodite. The maxilliped coxa, however, bears two well-developed endites (Endts) fringed with long hairs. Anatomically, the maxillipeds of Anaspides are the first pair of legs, but they are turned forward at the sides of the mouth parts in front of them (fig. 37 D) and are functionally a part of the feeding apparatus; according to Manton (1930), they are never used for walking or digging. In some Crustacea the next pair of legs, or the next two pairs, may also be transformed into maxillipeds.
In front of the maxillipeds are the two pairs of maxillary appendages (fig. 37 D, 1Mx, 2Mx), which, together with the maxillipeds (Mxpd), are suspended from the third, fourth, and fifth segments that have in common the single composite second tergal plate (III + IV + V). Both maxillae of Anaspides are small, much simplified appendages having no resemblance at all to a leg. Anatomically they are of interest chiefly as examples of the extent to which a segmental appendage may be reduced in size and simplified in structure. The second maxilla (fig. 38 F) consists principally of a large basal part, from the mesal margin of which projects a pair of hair-fringed endites (Endts), and which bears distally a movable, bilobed segment (Endpd) with two long brushes of hairs, which possibly represents the endopodite. The first maxilla, or maxillule (G), has a small basal segment (Cxpd) that appears to be the coxopodite, but the main body of the appendage presents on its posterior surface two large plates lying side by side, each of which is extended mesally into a broad endite (Endt). A small lobe (Endpd) on the outer side of the lateral plate may possibly be a remnant of the endopodite. It is evident that the parts of appendages such as the maxillae of Anaspides can be identified only when we have a series of forms that show the steps in the modification from a typical biramous limb. In some of the Crustacea the maxillae retain more distinctly a structure comparable with that of a leg, as will be seen in the crayfish, while in others they may be even more reduced than are those of Anaspides. The crustacean maxillae serve principally for passing food forward to the mouth.
Finally we come to the mandibles, which are the biting jaws of the animal. They are the first of the series of segmental appendages behind the head (fig. 37 D, Md) and correspond with the pedipalps of the Arachnida. The conversion of these appendages into jaws is distinctive of the crustaceans, the myriapods, and the insects, and separates these groups from the chelicerate arthropods, which have no true biting or masticatory organs.
The mandibles of Anaspides (fig. 37 D, Md) hang vertically from the tergum of the first body segment (II), on which each jaw has a single point of articulation. The body of each mandible (fig. 38 E, mdB) is broadly attached to the membranous lateral wall of its segment, and thus is freely movable on its tergal articulation (a). Visible evidence that the mandible is a modified leg is seen in the presence of a three-segmented palpus (Plp) arising from its outer surface, which evidently represents in reduced form the distal part of an ordinary limb. On the large basal part of the mandible (mdB) are attached the body muscles that move the appendage as a whole; these muscles correspond with the coxal muscles of a leg and thus show that the mandibular base is the coxopodite of the mandibular appendage. At its lower end, mesad of the palpus, the mandible is produced into a strong, free gnathal lobe (gnL), which is a specially developed coxal endite and is the effective part of the jaw. The gnathal lobe in Anaspides is differentiated into a thick basal molar process (mol) and a toothed distal incisor process (inc).
The musculature of the Anaspides mandibles is simple, but strongly developed (fig. 38 E). Each jaw has a small anterior dorsal muscle (da) and a large posterior dorsal muscle (dp), both arising on the tergum of the mandibular segment. Inasmuch as each mandible has a single dorsal point of articulation (a), and has little freedom of anterior and posterior movement, the dorsal muscles probably act as rotators of the jaw, but if they both pull together, they evidently may function also as adductors. The principal adductor muscles of the mandibles, however, are huge bundles of ventral fibers (v) filling the cavities of the mandibular bases and attached medially on a sheet of ligamentous tissue (Lg) suspended between the two jaws. These muscles of opposite sides pulling against each other effect a strong adductor action of the gnathal lobes. The intergnathal ligament when denuded of muscles (H) is seen to be a sheet of rather dense tissue expanded between the mandibles and connected posteriorly by two short arms with a second smaller ligament giving attachment to the maxillary adductors. The whole is hung from the back by three pairs of slender suspensory ligaments (slgs). The intergnathal ligament of lower Crustacea appears to be a structure of the same nature as the endosternum of the chelicerate arthropods, which gives attachment to the ventral muscles of the prosomatic appendages. Mandibles of the Anaspides type of structure appear to have no muscular mechanism of abduction: the jaws probably open by the elasticity of their basal connections.
The principal food of Anaspides is said by Smith (1909) and by Manton (1930) to be algal slime and organic detritus covering the rocks and plants on which the crustaceans live, but the latter eat also small animals such as the dead bodies of insect larvae or of their own species, and even small worms and tadpoles.
On the ventral surface of the maxillary region of the body (fig. 38 D) the sterna of the two maxillary segments are united in a single, median, deeply channeled sternal plate extending forward from the separate maxilliped sternum (VS) to the mouth. On each side the maxillary sternum gives off two intersegmental arms; one is an intermaxillary brachium (imB) between the bases of the first and second maxillae (1Mx, 2Mx), the other a postmaxillary brachium (pmB) separating the second maxilla from the maxilliped (Mxpd). On its anterior end the maxillary sternal plate supports two large, flat, divergent lobes (Pgn) lying against the posterior surfaces of the mandibles. These lobes are the paragnaths; they are characteristic organs of Crustacea, but they are not segmental appendages serially homologous with the other mouth parts and the ambulatory limbs; they probably belong to the mandibular segment. The median groove of the maxillary sternum runs into the mouth between the paragnath bases.
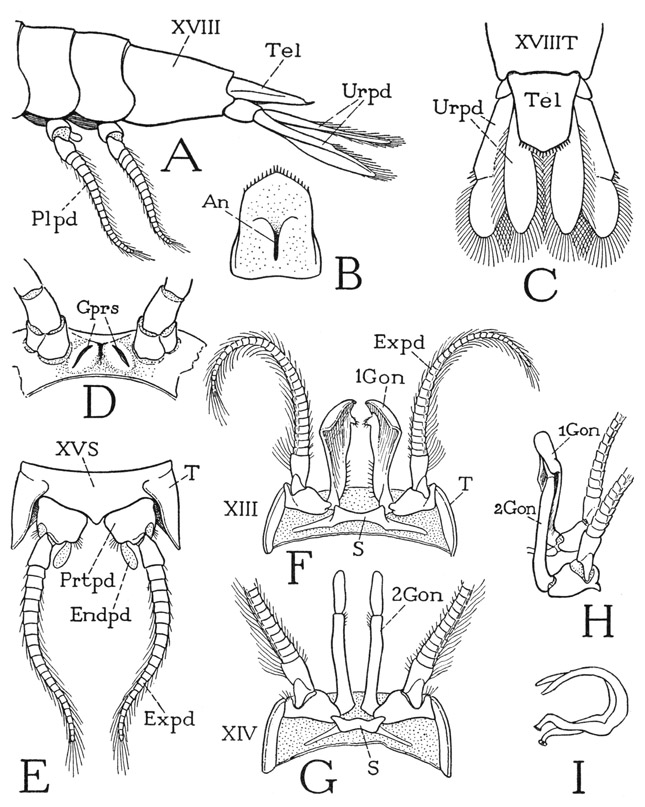
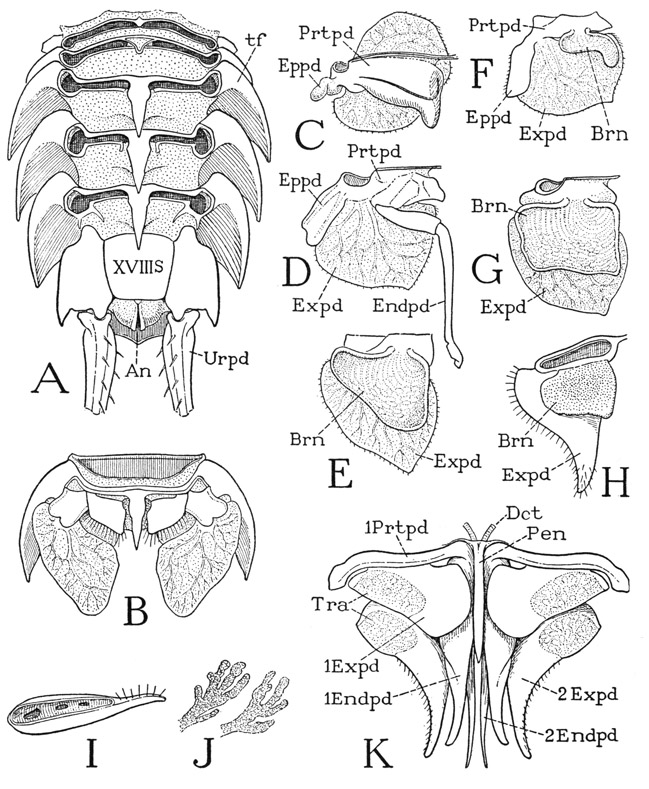
The abdomen of Anaspides is superficially distinguishable from the thorax by the shape of its first five tergal plates (fig. 37 A), the lower ends of which are expanded into broad, rounded lobes overlapping from before backward. Each of the corresponding five segments (XIII–XVII) bears a pair of pleopods (Plpds). The last segment (figs. 37 A, 39 A, XVIII) is much longer than the others, tapers posteriorly, and has no division between the tergal and sternal surfaces; its appendages are the uropods (Urpd). The body ends with the broad, flattened telson (Tel), which bears the anus on its undersurface (fig. 39 B, An).
The first five pairs of pleopods are all essentially of the same structure in the female; in the male the first two pairs are modified in form for reproductive purposes, but those of the third, fourth, and fifth segments are like the female pleopods, and any pair of these (fig. 39 E) may be taken to show the typical pleopod structure. The sternum (S) of the body segment is a broad plate lying anterior to the bases of the appendages and united laterally with the lobes of the tergum (T); between the appendage bases it is produced into a small median point. Each pleopod has a single, large basal segment (Prtpd), broadly attached on the posterior margin of the sternum and supporting laterally a long, many-jointed, hairy flagellum (Expd), which clearly corresponds with the exopodite of a thoracic limb. Mesally, the basal segment bears a small, soft lobe (Endpd), which is regarded as a much reduced endopodite, though its simple structure in itself would scarcely suggest this interpretation. However, the two parts being appended from the basal segment indicates that the latter is composed of both the coxopodite and the basipodite. A composite basal limb segment of this kind is termed a protopodite (Prtpd). On the fifth pleopods the endopodite lobe is either very small or absent.
The pleopods of the first and second abdominal segments of the male differ from those following and from the female pleopods in that each has a long, strong arm (fig. 39 F, G, Gon) from the inner end of the appendage base that projects forward beneath the thorax. The sternum of each of these segments is a small plate (S) lying behind the appendages, and produced on each side in a short postpedal extension. The mesal arms of the first two male pleopods evidently are the endopodites of the appendages, but they serve during mating for the transfer of sperm from the male to the female, and therefore may be termed gonapophyses. Those of the first pair (F, IGon) are relatively thick, widened distally, and their outer margins are folded ventrally and mesally to enclose deep grooves on the inner surfaces. The gonapophyses of the second pair (G, 2Gon) are slender processes, each divided into a long basal segment and a short distal segment. In the functional position (H) the end of each second gonapophysis is held in the groove of the corresponding first gonapophysis.
The spermatozoa of Anaspides are enclosed in two horseshoeshaped capsules, or spermatophores (fig. 39 I), formed in curved terminal parts of the genital ducts that open on the venter of the last thoracic segment (D, Gprs). The gonapophyses serve to introduce the spermatophores into the sperm receptacle on the sternum of the last thoracic segment of the female, but apparently the procedure has not been observed in Anaspides. Smith (1909), however, says that the spermatophores may sometimes be seen projecting from the receptacle of the female, and that they soon drop off after the spermatozoa have passed out of them. The openings of the oviducts, as already noted, are on the coxae of the fifth pair of legs of the female; the ova presumably flow posteriorly and are inseminated as they pass under the sperm receptacle. The female of Anaspides deposits her eggs on water plants, instead of carrying them on the pleopods as do many other Malacostraca.
The uropods, or pleopods of the sixth abdominal segment, consist each of two long, flat, hair-fringed lobes, an exopodite and an endopodite (fig. 39 C, Urpd), projecting posteriorly at the side of the telson from a small basal segment on the posterior angle of the last abdominal segment. The bases of the lobes are overlapped mesally by the telson, and the inner lobes overlap the somewhat larger outer lobes. The telson and the uropods together compose the so-called “tail fan” of the crustacean, which, however, is much more fan-shaped in the decapods (fig. 40) than in Anaspides.
Fig. 39. Crustacea—Anaspidacea. Anaspides tasmaniae Thomson.
A, distal segments of abdomen, lateral. B, telson, ventral. C, end of abdomen, dorsal, with uropods. D, seventh thoracic segment (XII) of male, ventral, with gonopores. E, third abdominal segment of male, ventral, pleopods turned back. F, first abdominal segment of male, ventral, with first pleopods. G, second abdominal segment of male, ventral, with second pleopods. H, first and second left pleopods of male, ventral, with gonopophyses in functional position. I, two spermatophores (from Smith, 1909).
For explanation of lettering see pages 190–192.
From observations on living specimens, Manton (1930) says that the entire body of Anaspides acts as a unit in locomotion to a much greater degree than is true with any other malacostracan, as might perhaps be inferred from its uniformity of structure. The first five pairs of pleopods are often used in conjunction with the legs during walking; and in swimming, the pleopods make the same movement that they do in walking. Anaspides, however, seldom swims freely except to go from one submerged rock or weed to another, but spends most of its time walking or half-swimming over the bottom in search of food and digging in the mud with its legs. The thoracic exopodites, Manton observes, are continually in motion, beating anteroposteriorly about 100 times a minute, and produce a backward flow of water along the sides of the body; the broad thin branchial epipodites beat in unison with the exopodites.
THE CRAYFISH, CAMBARUS
The crustaceans known as crayfishes are decapods of the group Astacura, which includes also the common lobster, Homarus, and a few species of other genera. Being widely distributed over the world, the fresh-water crayfishes have become favorite subjects for study in zoological courses. The genus Astacus of Europe and the western part of the United States is the usual crayfish of textbooks, but the crayfishes east of the Rocky Mountains in this country belong to several other genera, differing in certain respects from Astacus, and are grouped in the subfamily Cambarinae. Systematists recognize several genera and numerous species, but they are all so much alike in their general structure that we need not be particular as to the species, except with regard to the genital appendages of the male. The following descriptions and accompanying figures are based mostly on specimens of Cambarus longulus Girard, from St. Mary’s River in Virginia.
The name “crayfish” does not mean anything in itself. It is generally supposed to be a phonetic corruption of the French name for the animal, écrevisse, but Huxley (1880) suggested that it might as well have come from the Low Dutch name of crevik. The old English spelling was “crevis” or “crevise,” with the e pronounced like long a. The “vis” later became “fish” because the animal lived in the water and therefore should be a fish. Inasmuch, then, as “crayfish” has a devious etymological history and no claim to zoological correctness, the common American version of “crawfish” should be equally acceptable.
Fig. 40. Crustacea—Decapoda. Cambarus longulus Girard, male.
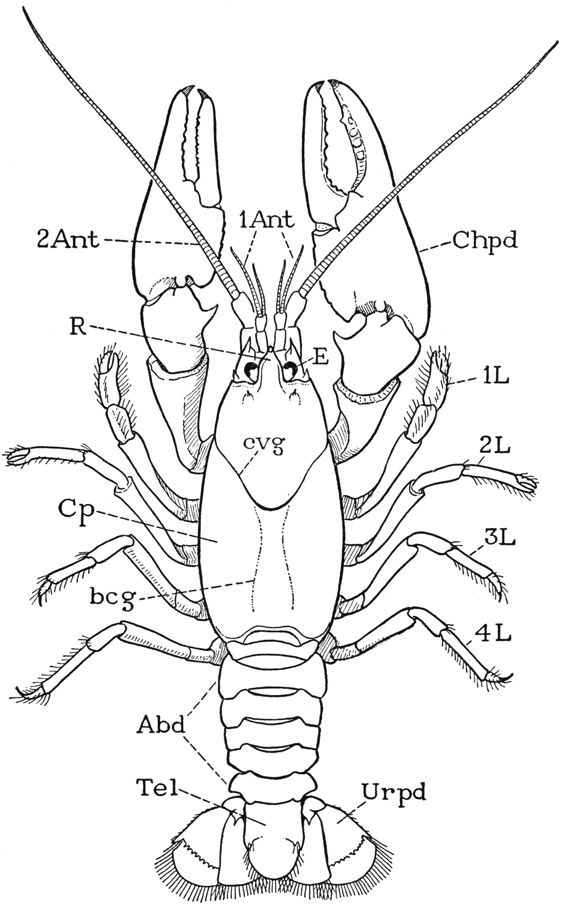
Abd, abdomen; 1Ant, first antenna, antennule; bcg, branchio-cardiac groove; evg, “cervical” groove; Chpd, cheliped; Cp, carapace; E, compound eye; 1L–4L, legs, second to fifth pereiopods; R, rostrum; Tel, telson; Urpd, uropod.
General External Features
The crayfish (fig. 40) gives a good example of the type of crustacean structure in which the gnathal and thoracic regions of the animal are covered dorsally by an unsegmented shell, called the carapace. On referring back to Anaspides (fig. 37), it will be seen that the carapace of the crayfish is a product of the union of at least six more tergal plates with the three already united in Anaspides. The part of the animal covered by the carapace is the gnathothorax, but the dorsum of the last thoracic segment appears not to enter into the composition of the carapace. Anteriorly the carapace of the crayfish is marked by a “cervical” groove (fig. 40, cvg) that sets off the mandibular region, which terminates in an apical rostrum (R). The lower edges of the carapace come down to the bases of the legs, but between them and the legs on each side is a long, narrow opening that leads up into a spacious cavity, the branchial chamber, which contains the gills. The gills arise from the coxal segments of the limbs, and also from the articular membranes above them. In front of the carapace, but mostly concealed beneath its projecting edge and the rostrum, is a distinct head structure, the protocephalon, very similar to that of Anaspides, which carries the stalked eyes, the two pairs of antennae, and the labrum. Behind the carapace is the more slender abdomen (Abd), consisting of six segments freely movable on the thorax and on one another. The abdomen ends with a broad, flat telson (Tel) having the anus on its undersurface. The abdomen of the crayfish thus differs little from that of Anaspides.
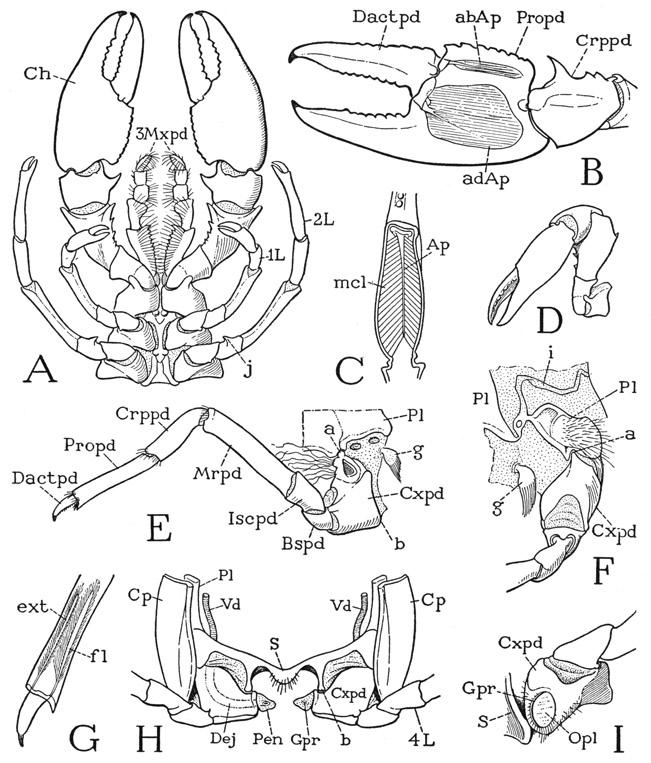
On the underside of the body, beneath the anterior part of the carapace are the usual three pairs of gnathal appendages, namely, the mandibles, the first maxillae, and the second maxillae. The next three pairs of appendages, instead of only the first pair as in Anaspides, serve as accessory organs of feeding, and are designated the first, second, and third maxillipeds. Since there are in all the same number of thoracic limbs in the crayfish as in Anaspides, the crayfish has only five pairs of pereiopods. The first pereiopods are specifically the chelipeds (fig. 40, Chpd) since they carry the large pincers, or chelae, of the crayfish; the next four pairs (1L–4L) are legs. The first and the second legs have small chelae, the last two pairs end with simple claws. Between the leg bases of opposite sides is seen the strongly sclerotized ventral wall of the thorax (fig. 43 A), in which all the sternal plates but the last are solidly united.
The six pairs of appendages of the abdomen resemble those of Anaspides, except that the first pair in the female crayfish are very small and unbranched. In the male the first two pairs are modified for reproductive purposes. The large lobes of the last pair, or uropods, form with the telson a broad tail fan. The female crayfish, unlike the female of Anaspides, does not deposit her eggs but carries them attached to the ventral pleopods.
Because of the structural differentiation of the several body regions of the crayfish, it is difficult to illustrate by any specific example the fundamental structure of a body segment. In the section on Anaspides it was observed that the back of each thoracic segment is covered by an arched tergal plate (fig. 41 A, T), but that a small laterotergal, or pleural, plate (Pl) intervenes on each side between the main dorsal plate and the base of the leg. The coxa of the leg is then articulated laterally (a) on the pleural plate, and mesally (b) on the sternum. A cross section of the thorax of a decapod (D) shows that the lateral articulations of the thoracic coxae are on the lower edges of plates (Pl) that form the inner walls of the gill chambers (brC). These plates, therefore, are the true lateral walls of the thorax of the crayfish, and correspond with the region of the laterotergal pleural plates of Anaspides. In Anaspides the gills project freely from the coxae of the legs (A, Brn); in the decapod (D) they are covered by long descending folds (tf) from the upper part of the carapace. A cross section of an abdominal segment (B) differs from that of the thorax in that the pleural areas (Pl) between the limb bases and the short tergal folds take a horizontal position in adaptation to the dorsoventral flattening of this part of the body. Again, in the maxillary region (C) the same parts (Pl) are also horizontal, forming strong lateral bridges between the bases of the second maxillae (2Mx) and the folds of the carapace (tf).
In the thorax of the crayfish the pleural and sternal regions of the united segments are joined by strong transverse bars between the bases of the limbs (fig. 41 F, icx). The segmental annuli of the abdomen are all separate from each other, and here it is seen that in each segment (E) the pleura and the sternum are united before and behind the limb bases by antecoxal and postcoxal pleurosternal connectives (acx, pcx). In the thorax (F) the union of the segments involves the pleurosternal connectives, so that the antecoxal connectives of one segment are united with the postcoxal connectives of the segment in front, and there is thus formed the series of intercoxal brachia (icx) separating the foramina of successive appendages. Each intercoxal brachium, however, is marked by a deep groove continuous on the one hand with the corresponding intersegmental groove between the united pleural plates, and on the other with that between the sterna. The endoskeleton of the decapod (G) is composed of pleural and sternal apodemes (plAp, sAp) inflected from the intersegmental grooves.
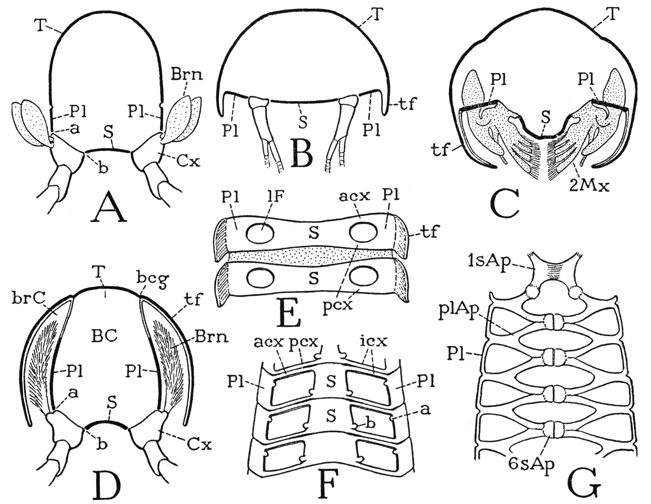
Fig. 41. Crustacea. Diagrams explanatory of decapod structure.
A, cross section of thoracic segment of Anaspides; pleura (Pl) are parts of dorsum carrying dorsal articulations (a) of coxae. B, cross section of astacuran abdomen, pleura horizontal and overlapped by tergal folds (tf). C, section of maxillary region; pleura form bridges between bases of maxillae and tergal folds. D, section of astacuran thorax, pleura vertical in lateral walls of body, long tergal folds (branchiostegites) extended downward to cover the gills. E, ventral surfaces of two consecutive abdominal segments, sternal and pleural regions connected by antecoxal (acx) and postcoxal (pcx) pleurosternal bridges. F, ventral surface of astacuran thorax, contiguous postcoxal and antecoxal bridges united, forming intercoxal brachia (icx). G, scheme of endoskeletal structure of the astacuran thorax, dorsal, composed of pleural (plAp) and sternal (sAp) apodemes arising from the intersegmental grooves.
For explanation of lettering see pages 190–192.
Carcinologists commonly describe a decapod as being divided into two tagmata, the first being the “cephalothorax,” which is mostly covered by the carapace, the second the abdomen. However, inasmuch as there is a distinct head section anterior to the carapace, and the region of the carapace includes only the segments of the feeding organs and the pereiopods, the cephalothorax really includes a head tagma and a gnathothoracic tagma. In the following account of the trunk regions of the crayfish, therefore, the tagmata will be described as the head (protocephalon), the gnathothorax, and the abdomen. There being the same number of trunk segments in the decapod as in Anaspides, the segments may be similarly numbered, beginning with the segment of the second antennae as segment I.
The Head
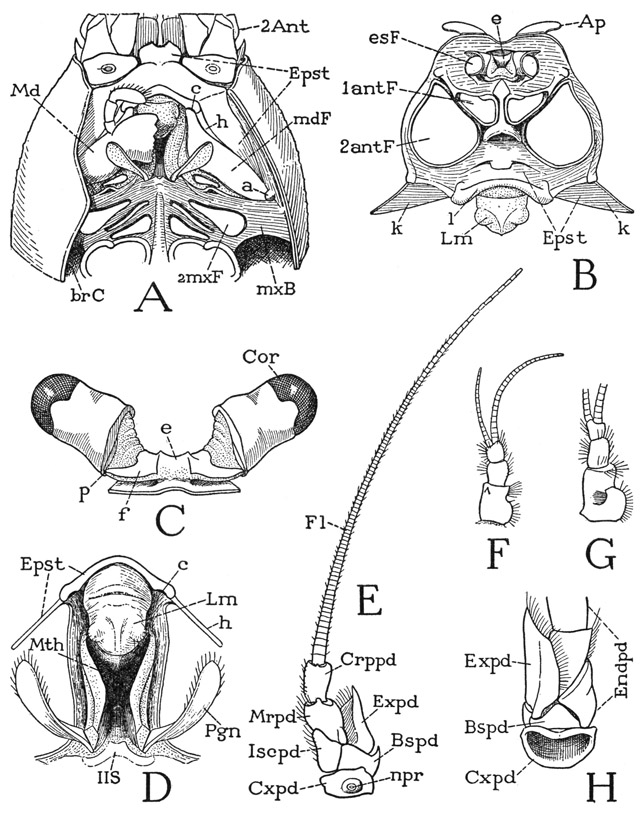
The head of the crayfish is a typical protocephalon; it has essentially the same structure as that of Anaspides, but it is more strongly calcified and the epistomal region is more elaborately developed. Viewed anteriorly, with the appendages removed, the head skeleton (fig. 42 B) presents dorsally a broad surface perforated by the foramina of the eyestalks (esF), and reflected posteriorly into the underfolded margin of the carapace. From the groove between the head and the carapace arises a pair of flat, divergent apodemes (Ap), on which are attached broad muscles going to the dorsal wall of the proventricular “cardiac” sack of the stomodaeum. Below the ocular region mesally are the foramina of the first antennae (1antF), and laterally the huge foramina of the second antennae (2antF). Between the antennae is a narrow, vertical frontal ridge ending ventrally in a protruding lobe on the upper angle of the epistome (Epst). The epistome expands beneath the antennae and becomes continuous laterally with narrow bars ascending along the sides of the antennal foramina that connect the epistome with the ocular region of the head. The median part of the epistomal margin carrying the labrum (Lm) is thickened to form a strong supralabral ridge (l), laterad of which the epistome is produced on each side into a triangular, winglike posterior extension (k) that unites laterally with the base of the inner lamella of the carapace fold.
Fig. 42. Crustacea—Decapoda. Cambarus longulus Girard. The head and the protocephalic appendages.
A, head and anterior part of body, ventral, left mandible, maxillae, and maxillipeds removed, fold of carapace cut off on left side. B, skeleton of head, anterior. C, eyestalks and attachment on dorsal wall of head. D, mouth region, with labrum and edge of epistome, ventral. E, left second antenna, ventral. F, left first antenna, ventral. G, right first antenna, dorsal. H, base of left second antenna, dorsal.
For explanation of lettering see pages 190–192.
In the front view of the head (fig. 42 B) the epistomal region is foreshortened because it turns posteriorly from beneath the antennae; its true form is better seen in the ventral view shown at A of the figure, which, however, is taken from another species. Though the epistome is inflected upon the ventral surface of the crayfish, it is a preoral structure since it carries the labrum, which projects over the mouth behind it (D, Mth). The epistome, therefore, is not a sternal plate either of the second antennal segment or of the mandibular segment, since these segments are primarily postoral. The epistome belongs to the protocephalon, and in most arthropods, including the amphipods and isopods among the Crustacea, it has a facial position (fig. 49 B). The strong development of the epistome in the decapods is correlated with the fact that the mandibles are articulated mesally on it at the ends of the supralabral ridge (fig. 42 A, c) and are hinged on the posterior margins (h) of the epistomal wings. These connections of the mandibles with the epistome have been secondarily evolved in the decapods; they are not present in Anaspides or the entomostracan crustaceans. The persisting primary articulations of the mandibles are those on the carapace (a).
The Gnathothorax
In a study of the gnathothoracic region of the body, it will be convenient to consider separately the carapace, the branchial chambers and the pleura, the mouth, the ventral skeleton, and the pleurosternal endoskeleton.
The Carapace— The carapace is composed of the united terga of at least the first ten body segments behind the head. The last component tergum probably pertains to the seventh thoracic segment, for, though there is no individual tergal plate corresponding with the eighth thoracic segment, this segment appears to make no contribution to the carapace, and its pleural and sternal parts are not united with those of the segments before it. The anterior part of the carapace is set off by a U-shaped groove on the back (fig. 40, cvg) that runs downward and forward on the sides. This impression is commonly termed the “cervical groove,” and the part of the animal before it is regarded as the “head,” which is supposed to include the segments of the mandibles and the two pairs of maxillae. The dorsal muscles of the first and second maxillae, however, are shown by Schmidt (1915) in Astacus to have their origins on the carapace behind the groove, while the mandibular muscles arise in front of it. The part of the carapace before the groove in the crayfish, therefore, clearly corresponds with the first free tergal plate of Anaspides (fig. 37 A, D, II), which supports only the mandibles, the maxillary terga of Anaspides being combined with the tergum of the first thoracic segment. However, since the tergal region before the “cervical groove” gives attachment also to the dorsal muscles of the second antennae, it might be suspected of including the dorsal arc of the second antennal segment. Other intersegmental lines are entirely obliterated in the carapace of Cambarus, but the dorsum behind the “cervical groove” is marked by a pair of faintly impressed, sinuous, longitudinal lines called the branchiocardiac grooves (fig. 40, beg). On the sides of the animal the carapace curves downward and ends with free margins close to the bases of the appendages. The entire margin on each side is fringed with fine, closely set hairs.
The Branchial Chambers and the Pleura— The open space on each side of the body between the edge of the carapace and the bases of the pereiopods leads upward, as has already been noted, into a large chamber that contains the gills (fig. 41 D, brC). The outer wall of each branchial chamber is soft and membranous, and is reflected upward directly from the edge of the hard outer wall of the carapace, on which it is closely adnate. When this inner wall of the carapace fold (tf) reaches the site of the branchiocardiac groove (bcg) on the dorsum, however, it becomes free and turns downward to unite with the upper edge of a long plate (Pl) that forms the lower part of the inner wall of the gill chamber and supports the appendages. The inner-wall plates of the gill chambers are thus seen to be the true lateral walls of the thorax covered by folds of the carapace that have grown down over the gills. They are commonly termed “epimera” by carcinologists, but we have already given reasons for calling the plates in question the pleura, because they so evidently correspond with the laterotergal, or pleural, plates of Anaspides (A, Pl) and with the sclerotic lateral areas known as the pleura in other arthropods.
Fig. 43. Crustacea—Decapoda. Cambarus longulus Girard. The gnathothorax.
A, ventral view of gnathothoracic region of body of male, with appendages removed. B, pleural wall of left branchial chamber. C, a right pair of pleural and sternal apodemes, dried until parts completely separated, showing interlocking fimbriated margins, mesal view. D, section of first leg segment, anterior. E, ventral surfaces of segments XI and XII of female, with annulus ventralis. F, vertical section of lower part of segment of second legs of male, showing relation of pleural and sternal apodemes, anterior. G, ventral gnathothoracic endoskeleton, dorsal.
For explanation of lettering see pages 190–192.
The branchial chambers extend forward from the posterior end of the carapace into the segment of the second maxillipeds (fig. 43 A), which bear the first gills (fig. 44 G). When the interior of a branchial chamber is exposed, it is seen that the pleural plate on the inner wall (fig. 43 B) extends from the second maxilliped segment (VI) into the segment of the fourth pair of pereiopods and is marked by five intersegmental grooves showing that it is formed by the union of the pleura of six successive segments. The corresponding limbs articulate on small knobs (a) on the lower margins of their respective pleural areas. Behind this composite pleural plate is a second smaller plate (XII) in the pleural wall of the last thoracic segment, which supports the fifth pereiopod, but there are no gills on this segment.
The major pleural plate of the gill chamber (fig. 43 B) has a rounded upper margin with a notch behind the middle and is continuous with the membranous integument above it reflected from the inner wall of the carapace fold. The lower margin is somewhat thickened and scalloped over the limb bases. Deep slits in the lower ends of the intersegmental grooves (inv) mark the roots of the pleural apodemes of the thorax (G, plAp). Between the limb bases the pleuron is connected with the sternum by intercoxal pleurosternal brachia (A). Anteriorly the pleural plate (B) enlarges upward in the maxilliped region, creating here a pocketlike dorsal expansion of the gill chamber. From the anterior wall of the pocket the pleural sclerotization becomes horizontal and forms a strong bridge between the base of the second maxilla and the inner wall of the carapace fold (fig. 41 C, Pl). In the maxillary region is the pumping apparatus of the respiratory system that creates a water current through the gill chambers, but this structure and the gills themselves will be described in connection with the appendages and the general respiratory system.
The pleural plate of the last thoracic segment is a small, oval plaque (fig. 43 B, XII), to which the fourth leg is articulated (fig. 45 F). Its posterior setose part is somewhat exposed beyond the end of the carapace and is strongly connected with the sternum of the segment by a postcoxal pleurosternal arm. Above this pleural plate the membranous integument of the last thoracic segment contains a curious angulated bar (i) that connects the thoracic pleuron with the abdomen. The bar begins between the adjacent ends of the two pleural plates of the thorax as a small expansion, narrowly connected with each plate, and then proceeds upward and posteriorly over the second plate as a ridge on the inner surface of the membranous integument, which finally connects with the anterior margin of the first abdominal tergum.
The Mouth— The mouth of a crustacean lies immediately behind the cephalic epistome and between the bases of the mandibles. In the Malacostraca it is limited posteriorly by a small plate at the anterior end of the ventral skeleton that appears to be a remnant of the mandibular sternum. The segmental status of the mouth is uncertain since there is nothing in the adult animal that has been identified with the sternal arc of the postoral embryonic segment of the second antennae. For practical purposes the mouth must be between the jaws, and probably it has acquired this position secondarily, while the mandibular sternum has been either deleted or transposed to make way for the mouth.
The mouth of the crayfish (fig. 42 D, Mth) is a large, elongate, distensible opening above the gnathal lobes of the mandibles. The labrum (Lm) projects below its anterior end, and a ridge on the metastomal plate (IIS) guards its posterior end. Laterally the mouth is bounded by two thick integumental folds, from the posterior ends of which arise the long, flat paragnaths (Pgn). The mouth folds are separated from the bases of the mandibles by strips of flexible integument. The size and shape of the mouth vary with the separation or approximation of the lateral folds, but the oral aperture leads into a deep, funnel-shaped cavity, regarded as the oesophagus, at the inner end of which may be seen the smaller opening into the proventricular sac of the stomodaeum known as the “cardiac chamber of the stomach.” A thick fold on the anterior wall of the oesophagus proceeds inward from the labrum.
The Ventral Skeleton— The entire undersurface of the gnathothoracic region of the crayfish contains a strongly developed ventral sclerotization composed of the segmental sterna and the pleurostemal connectives (fig. 43 A). At the anterior end of the ventral skeleton is the small metastomal plate (IIS), which is the only part that might be referred to the mandibular segment. From the metastomal plate a long median sternal bar extends posteriorly between the bases of the second maxillae, the three pairs of maxillipeds, and the first pair of pereiopods (Chpd) and gives off on each side a series of brachia separating the foramina of the appendages. The small first maxillae (1Mx) arise from the membranous areas behind the mandibles, but they are separated from the foramina of the second maxillae by the wide first pair of sternal brachia (imB), which contain the pits (1inv) of the first sternal apodemes, showing that these brachia include the postcoxal elements of the first maxillary segment. The second maxillae have a lateral position, but the intermaxillary brachia and the postmaxillary brachia unite laterad of them in wide bridges (fig. 42 A, mxB) that join the inner lamellae of the carapace folds and form the anterior limits of the branchial chambers. These maxillary bridges, as already explained, are the pleural plates of the maxillary segments (fig. 41 C, Pl); posteriorly they are continuous with the pleura of the thoracic segments (D, Pl), which abruptly assume a vertical position on the inner walls of the gill chambers. The foramina of the three pairs of maxillipeds (fig. 43 A, 1Mxpd, 2Mxpd, 3Mxpd) are crowded forward between the widely separated foramina of the second maxillae (2Mx), so that the outer ends of the coxae of the second maxillipeds, which carry the first gills, come to be opposite the anterior ends of the branchial chambers.
On the body segment of the first legs (fig. 43 A, 1L) the sternal sclerotization widens posteriorly and becomes successively broader and deeply concave on the following two segments. The posterior angles of each segmental area of the composite thoracic sternum are produced into knobs that bear the sternal articulations of the coxae (b). Since the lateral articulations (a) are anterior on the corresponding pleural areas, the axes of the coxae on this part of the body are transversely oblique, almost at an angle of 45 degrees. Intersegmental pleurosternal brachia separate the foramina of the appendages back to the third pair of legs, but there are no pleurosternal connections between the third and fourth legs.
The sternum of the last thoracic segment (fig. 43 A, XIIS) is an entirely distinct plate between the bases of the fourth legs, separated from the sternum in front of it by flexible integument. It is connected by postcoxal arms with the pleural plates of its segment (fig. 45 H), and the coxal axes of the last legs are directly transverse between the pleural and sternal articulations. This last sternal plate of the thorax differs in shape in the male (fig. 43 A) and the female (E).
On the posterior part of the thoracic sternal region in the female of crayfishes belonging to the Cambarinae, but not in Astacus, there projects posteriorly from the sternum of the seventh segment a thick circular lobe partly overlapping the sternum of the eighth segment (fig. 43 E, Anv). This structure is known as the annulus ventralis; it contains a small sac, which is the sperm receptacle, or spermatheca, of the female. The ventral surface of the annulus is concave between two rounded marginal elevations, separated posteriorly by a suturelike groove. From one side a prominent ridge traverses the ventral concavity of the organ and ends below the marginal thickening of the other side, in some specimens going to the right, in others to the left. Just behind the ridge is a narrow slitlike aperture opening into a hard-walled spermathecal sac lying transversely within the annulus. An elaborate description of the annulus ventralis and sperm receptacles in several cambarine species is given by Andrews (1906, 1908).
During mating, the male crayfish introduces the spermatozoa into the spermatheca of the female by means of the genital processes on his first and second abdominal appendages, the structure of which will be described in the section on the appendages; but it is difficult to understand how the sperm is discharged by the female at the time the eggs are liberated. Andrews (1906), however, gives evidence that the sperm may be ejected by pressure resulting from a forcible retraction of the free last thoracic sternum against the annulus ventralis. The openings of the oviducts are on the mesal ends of the coxae of the third pereiopods of the female (fig. 45 I, Gpr), and beyond them the sternal concavity forms a channel widening posteriorly to the end of the seventh segment, where the annulus ventralis is situated. Andrews (1906) records observing the discharge of the eggs in two semiliquid streams that flow down the sternal channel, as the female lies on her back with the abdomen bent forward. Presumably the eggs are inseminated when they reach the annulus ventralis, and are then attached to the abdominal pleopods.
The Endoskeleton— The decapod crustaceans have an elaborately developed endoskeleton extending from the maxillary segment to the last thoracic segment. It is composed of intersegmental pleural and sternal apodemes, most of which in the Astacura unite over the ventral nerve cord in a series of transverse bridges that support the alimentary canal and other viscera above them and give attachment on their undersurfaces to the ventral muscles of the appendages.
The endoskeleton of the crayfish, as seen from above (fig. 43 G), appears to consist of five horizontal median plates each supported on each side by a pair of convergent arms from the pleural wall of the gill chamber. In the seventh and eighth thoracic segments, however, the endoskeletal elements are entirely sternal (7sAp, 8sAp). When a specimen is thoroughly cleaned and dried, it is seen that each apparent median plate of the endoskeleton is divided along the middle and that the lateral arms are not extensions of the plates but are joined to them by interlocking fimbriations. Moreover, each half-plate is merely the expanded upper end of an intersegmental sternal apodeme, and the anterior and posterior arms attached on successive plates are seen to branch from a common stalk arising from an intersegmental groove of the pleuron. The anatomical components of the endoskeleton, therefore, from the second maxilliped segment to the segment of the third pereiopods, are a series of horizontal, Y-shaped intersegmental pleural apodemes along each side, and a double row of vertical, T-shaped sternal apodemes. The converging arms from successive pleural apodemes are united with the sternal apodemes between them by a mutual interlacing of their irregular, fimbriated opposing margins, and the plates of the two adjoining sternal apodemes are united with each other. At C of figure 43 is shown in mesal view the pleural and sternal apodemes of the right side arising on the intersegmental line between the fifth and sixth thoracic segments, after having been cleaned and dried until the associated parts have become entirely disconnected. In this condition the highly irregular shapes and frayed-out margins of the pleural arms and the sternal plates become very apparent. The long anterior arm of the sternal apodeme was not observed in the other segments.
The bases of the pleural and sternal apodemes arise not only from the intersegmental grooves of the pleura and sterna, but they invade the intercoxal pleurosternal brachia between them and thus become confluent with each other (fig. 43 F). In a cross section of the thorax (F) showing a pair of sternal apodemes and the corresponding anterior arms of the pleural apodemes behind them, the endoskeletal complex looks like a vertical pleurosternal plate perforated by a large median foramen and two smaller lateral foramina. The median bridge between the stalks of the sternal apodemes is termed the mesophragm of the endoskeleton, the lateral parts the paraphragms.
The anteriormost component of the ventral endoskeleton is the so-called “head apodeme.” It consists of a pair of sternal apodemes arising from the intermaxillary sternal brachia, the roots of which are marked externally by conspicuous pits in front of the second maxillae (fig. 43 A, 1inv). The two apodemal plates are firmly united by long, interlacing strands from their opposed margins, and thus form a broad median bridge (G, 1sAp) supported on the lateral stalks. The posterior angles of the bridge are united with the anterior arms of the first pleural apodemes (1plAp), which arise between the second and third maxilliped segments, and also with a pair of small, irregular plates (2sAp) of the sternal apodemes of the intercoxal brachium between the first and second maxillipeds.
Between the segments of the last two pairs of legs (fig. 43 G, XI, XII) the only representative of the pleural apodemes is a small spur arising from the inner surface of the expanded end of the thoracico-abdominal connective rod inserted between the seventh and eighth thoracic pleura. Sternal apodemes, on the other hand, are present in both the seventh and eighth segments of the thorax. Those of the seventh segment (7sAp) are a pair of broad plates diverging forward and united by their anterior margins with the preceding en-dostemal plates and the posterior arms of the last pleural apodemes. The sternal apodemes of the eighth segment are crestlike lobes (8sAp) arising directly from the sternum and have no connection with the rest of the endoskeleton.
The Abdomen
The six-segmented abdomen of the crayfish tapers from the thorax to the telson; its dorsal surface is transversely rounded, the ventral surface somewhat concave. The segments are continuously calcified annuli, separated from each other, as is the first segment from the thorax, by flexible intersegmental conjunctivae. At its base the abdomen is freely movable on the thorax because it is yoked to the latter only by the pair of long slender bars, already described (fig. 45 F, i), that arise between the two pleural plates of the thorax and curve posteriorly in the membranous body wall over the second plates to attach on the anterior margin of the first abdominal tergum. The successive segmental rings of the abdomen, however, are firmly hinged on each other by paired articular knobs on the anterior tergal margins (fig. 46 B, r), which limit the movements of the segments on each other to motion in a vertical plane.
The arched tergal region of an abdominal segment (fig. 46 B, T) is produced on the sides in expansions that form free lateral lobes overhanging the undersurface of the segment. Anteriorly each tergal arch is extended into a smoothly convex, crescent-shaped area that glides under the tergum in front when the abdomen is straightened but becomes fully exposed when the abdomen is deflexed. The sternum of an abdominal segment is the narrow ventral part of the segmental annulus between the bases of the pleopods (B, C, S). Laterally, except on the last segment, the sternum expands and divides into antecoxal and postcoxal arms that unite laterad of the appendages with the pleural areas of the segment (Pl) mesad of the tergal lobes. On the second to the fifth segments the pleural areas are hardly to be distinguished from the overhanging lobes of the tergum, but on the first segment of the female (A) the small pleopods are relatively close together, and long pleural areas (Pl) intervene between them and the tergum. Because of the continuity of the sclerotization in the walls of the abdominal segments, however, there is only a topographical distinction between tergal, pleural, and sternal regions. The last abdominal sternum (E, XVIIIS) is a wider plate than the others, and it does not directly carry the uropods, which arise from a membranous integument behind the sternum and are pivoted on slender processes of the sternal margin. The terminal telson (D, Tel) is a flat lobe with a transverse line of flexion across its middle; the anus (E, An) is on the ventral surface of the proximal part.
The Appendages
In a restricted technical sense the “appendages” of an arthropod are the paired limbs that pertain to individual trunk segments, and presumably are serially homologous. The eyestalks and the parag-naths of the Crustacea are not generally regarded as true appendages according to this definition, and the status of the first antennae as segmental appendages is uncertain. Yet these organs are appendicular structures and as such will be included under the present heading.
The Eyestalks— Inasmuch as the head of the crayfish is buried beneath the front edge of the carapace and the rostrum, it is quite necessary that the eyes should be elevated on stalks, and, being on stalks, it is a further advantage to have the stalks movable. Crustaceans in which the head is fully exposed usually have sessile eyes. Eyestalks are present in most of the Malacostraca, but in the malacostracan amphipods and isopods, as in most other arthropods, the eyes are on the head surface.
The eyestalks of the crayfish (fig. 42 C) arise from the sides of a small median elevation, or ocular lobe, on the dorsal wall of the head (B, e). Each stalk has two parts generally termed segments, but the proximal “segment” is merely flexible on the supporting head lobe and has no muscles inserted on it; the large distal segment, however, is freely movable on the basal segment and is amply supplied with muscles arising in the latter. The proximal segment is funnel-shaped, expanding outward from its narrowed base, and is largely membranous except for a dorsal sclerotization of its basal part, which posteriorly is prolonged into an arm (C, f) on which the movable distal segment is pivoted (p). The large, fully sclerotized distal segment is somewhat bell-shaped, with its rounded outer end capped by the faceted cornea (Cor). It should be noted that the facets of the crustacean compound eye are square, not hexagonal as in insects. The eye-bearing segment can be turned in any direction on the supporting arm of the basal segment; in Astacus, Schmidt (1915) describes seven ocular muscles between the two segments of each eyestalk. In addition, the stalks may be moved by a pair of muscles that are inserted on the ocular lobe of the head and that arise on a long tendon from the epistome. The contraction of these muscles, according to Schmidt, produces an infolding of the flexible anterior wall of the lobe, which in turn somewhat elevates the attached stalks and directs them more anteriorly.
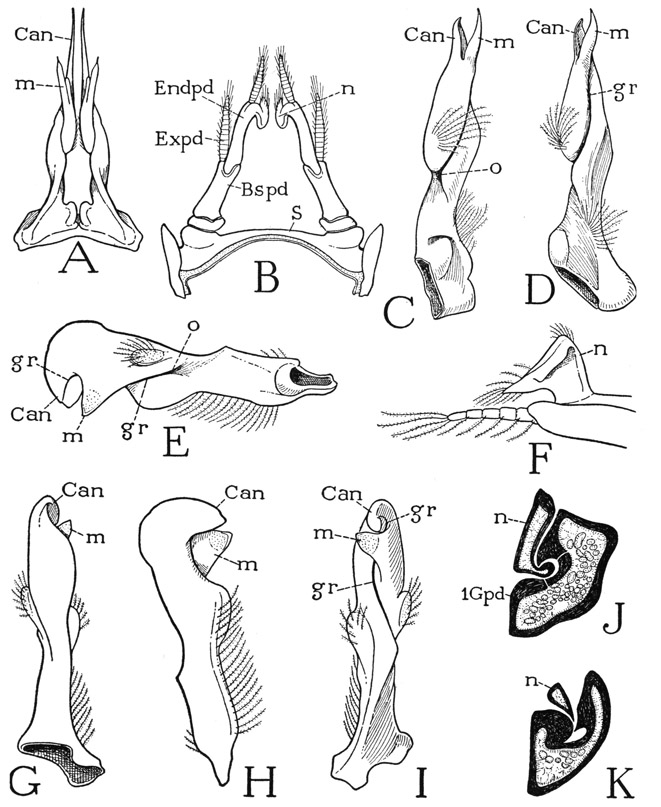
The First Antennae, or Antennules— The first antennae are relatively small appendages (fig. 42 F) arising close together on the head beneath the eyestalks and at the sides of the narrow frontal bar (B, 1antF). Each antennule (F) consists of a basal stalk of three segments and a pair of slender, many-jointed distal flagella, of which the outer flagellum is the longer. The basal muscles of the antennules are said to arise on the rims of the antennular foramina of the head, and they thus pertain to the protocephalon. The dorsal surface of the basal segments of each appendage is slightly concave and lies just beneath the corresponding eyestalk; in the depression is a crescentic slit, the outer lip of which is fringed with long hairs (G). The slit is the opening of a pocket, known as the statocyst (formerly thought to be an auditory organ and called the “otocyst”). The walls of the cyst are provided with flexible, innervated setae, and the cavity contains minute particles termed statoliths, which in most of the decapods are grains of sand introduced by the animal itself with its chelae just after moulting, but in some the grains are said to be crystals of calcium carbonate secreted within the cyst. By the secretion of glands beneath the bases of the setae the statoliths become attached to the setae, and their movements are supposed to orient the animal to gravity. According to Prentiss (1901), if iron filings are substituted for the statoliths in a shrimp, the animal will respond to the attraction of an electromagnet.
The Second Antennae— If the second antennae are appendages of a primarily postoral somite of the embryo, as they are said to be, they should belong to the series of body limbs, and, in fact, they seem to show a relationship to these appendages in their structure. The basal muscles of the second antennae arise on the carapace, except one that comes from the epistome. The shaft of each antenna (fig. 42 E) consists of five distinct segments, the last of which bears a single, long, multiarticulate flagellum, probably representing a sixth segment, since a pair of muscles is inserted on the base of the flagellum, while there are no muscles within the antennal flagellum itself. From the second segment beyond the base (E, Bspd) there arises laterally a broad lobe (Expd), which evidently represents the exopodite of a biramous limb, so that the rest of the shaft appears to be a four-segmented endopodite. The horizontal joint between the third and fourth segments allows a free up-and-down movement to the part of the antenna beyond it, but, since the axis of this joint is very oblique, when the antenna is flexed ventrally, the flagellum turns over to the opposite side of the body. The fifth segment flexes horizontally on the fourth, principally in a lateral direction, and the flagellum has a free movement on the fifth segment in a horizontal plane. On the ventral surface of the coxopodite is a conspicuous aperture, the nephropore (E, npr), which is the exit of the antennal excretory gland. On the dorsal surface of the antennal base (H) the coxal rim is very narrow, and the basipodite appears only as a triangular plate bearing the endopodite and the exopodite.
The Mandibles— The mandibles are the appendages next in order according to their position, but since they are covered from below by the several pairs of appendages immediately following them, which in turn underlap each other, the mouth parts of the crayfish cannot conveniently be studied in the order of their succession as here described. The student, therefore, is advised to turn first to the third maxillipeds and to work forward from them, then returning to the pereiopods.
The transversely elongate mandibles of the crayfish (fig. 42 A, Md) lie between the epistome (Epst) and the pleural bridges of the maxillary segment (mxB), where they extend obliquely forward and somewhat downward from the bases of the inner walls of the carapace folds to the mouth. They are strongly hinged on the lateral wings of the epistome (h) but are separated from the maxillary bridges by membranous areas that contain the first maxillae. The mandibles are closely embraced by the paragnaths behind them and are covered ventrally by the endites of the first and second maxillae and the first maxillipeds.
Each mandible (fig. 44 A) consists of a broad, quadrate basal part attached on the mandibular segment and of a large, free, bluntly toothed gnathal lobe (gnL) that projects below the mouth. At the base of the lobe arises anteriorly a three-segmented palpus (Plp). The posterior lateral angle of the mandible (a) is articulated to the inner wall of the carapace fold in the angle between the carapace and the maxillary bridge (fig. 42 A, a). The anterior mesal angle of the mandibular base is produced into a large process (fig. 44 A, c) by which the jaw articulates on the epistome at the side of the base of the labrum (fig. 42 A, D, c). The mandibular axis of movement, therefore, is oblique between these two points of articulation, and along this axis the anterior margin of the jaw is strongly hinged on the lateral wing of the epistome (h). The movement of the mandible is thus strictly limited to a partial rotation on the axis (fig. 44 A, a–c) between the carapace and the epistome; consequently the gnathal lobe turns only up or down on the radius c–d from the mesal articulation c. The two jaws, therefore, open and close from below like a pair of valves.
When the mandible is removed from its basal connections, it is seen that the body of the jaw has a deep inner cavity (fig. 44 B) continuous with the haemocoele of the body. The epistomal hinge (h) falls in line with the axis (a–c) between the articulations, but laterad of the axis the anterior margin of the jaw is produced dorsal to the hinge line in a large, triangular apodemal extension (A, B, Ap). The gnathal lobe has a smooth inner surface, but at its base are two thick prominences that serve for crushing rather than mastication.
The basal musculature of the mandible includes six muscles (fig. 44 B), three (1, 2, 3) attached on the anterior margin of the jaw, one (4) on the posterior margin, and two (5, 6) within the basal cavity. The first two anterior muscles (1, 2) arise laterally on the carapace and are inserted on the mandibular apodeme. These muscles, therefore, are anterior dorsal adductors, since they pull outward on the apodeme above the hinge line of the jaw. The third muscle of the anterior group (3) arises on the carapace dorsal to the mandible and is a small dorsal abductor. The posterior muscle (4) is the largest of all the jaw muscles; its fibers arise in a huge conical bundle on the dorsal wall of the carapace anterior to the “cervical groove” and are inserted on a long thick tendon attached to the posterior margin of the mandible close to the base of the gnathal lobe. This muscle is a powerful posterior dorsal adductor of the jaw. The two muscles inserted within the mandibular cavity arise on the endosternal “head apodeme” of the maxillary region. The larger of the two (5) is a thick bundle of fibers that spread out into almost the whole interior of the mandible and constitute a ventral adductor of the jaw. The other, smaller muscle from the endostemum (6) goes to the inner face of the mandibular apodeme above the hinge line of the jaw and is thus a ventral abductor directly opposed to the two dorsal adductors (1, 2). In a more generalized mandible, such as that of Anaspides (fig. 38 E), having only a dorsal point of articulation, the three anterior muscles of the decapod jaw are represented by the single dorsal promotor, or anterior rotator, and the ventral fibers of both jaws are united in a common intergnathal adductor. The dorsal adductor of Cambarus (fig. 44 B, 4) is the dorsal remotor, or posterior rotator, of the Anaspides mandible.
The Paragnaths— The paragnaths are flat, elongate, somewhat spatulate lobes arising from the posterior ends of the lateral mouth folds (fig. 42 D, Pgn); they are closely applied to the undersurfaces of the mandibles. The paragnaths have no musculature and, according to Keim (1915), are innervated from branches of the mandibular nerves. Between their bases is a small metastomal plate (IIS), possibly a remnant of the mandibular sternum.
Fig. 44. Crustacea—Decapoda. Cambarus longulus Girard. The mouth parts and maxillipeds.
A, left mandible, ventral. B, left mandible and its muscles, dorsomesal. C, left first maxilla, ventral. D, left second maxilla, ventral. E, right second maxilla, dorsal. F, right first maxilliped, dorsal. G, left second maxilliped, lateral. H, left third maxilliped, lateral.
For explanation of lettering see pages 190–192.
The First Maxillae— The very small first maxillae, or maxillulae, are situated in the membranous integument behind the mandibles at the sides of the mouth (fig. 43 A, 1Mx) and have no connection with the ventral skeleton. Each appendage (fig. 44 C) appears at first inspection to be an assemblage of three thin, flat lobes with hairfringed margins closely applied to the undersurface of the gnathal lobe of the corresponding mandible. In the isolated appendage, however, it is seen that the lobes are supported on a basal coxopodite (Cxpd). The first lobe (1Endt) is carried on an arm from the coxopodite and is evidently a coxal endite. The other two lobes are borne on a second arm (Bspd), which may be regarded as the basipodite carrying a basal endite (2Endt) and a much reduced endopodite (Endpd). On its anterior (dorsal) surface the endopodite is armed with a strong basal tooth.
The Second Maxillae— The maxillary appendages of the second pair (fig. 44 D), often called simply the maxillae, are somewhat larger than the first maxillae and have a more lateral position than any of the other mouth parts. They arise from large foramina of the ventral skeleton at the sides of the first maxillipeds (fig. 43 A, 2Mx). Each appendage carries two long, bifid, densely fringed endite lobes (fig. 44 D, Endt), a slender, tapering endopodite (Endpd), and a long, flat, lateral lobe (Scpg), known as the scaphognathite presumably from its fancied resemblance to a boat. The segmentation of the maxilla is obscure, but the basal part (Cxpd) carrying the first pair of endites must represent the coxopodite. The distal pair of endites and the endopodite arise from a common basal part (Bspd), which therefore should be the basipodite. The endites project forward and mesally from the basal region of the maxilla and underlap the endites of the first maxilla.
The long scaphognathite of the second maxilla (fig. 44 D) is attached near its middle to the base of the appendage and extends horizontally forward and backward. Its more slender anterior lobe lies against the undersurface of the maxillary bridge of the ventral skeleton and beneath the lateral part of the mandible; the shorter and broader posterior lobe projects into the anterior part of the branchial chamber. The maxillary scaphognathites are vibratory organs that cause the forward flow of water through the gill chambers. Each organ in the European crayfish, Astacus, is shown by Schmidt (1915) to be provided with eight muscles, one arising in the basipodite of the maxilla, the other seven on the maxillary pleuron and on the maxillary apodeme of the endoskeleton. The seven body muscles enter the base of the maxilla through a cup-shaped opening on the dorsal side (fig. 44 E). The same musculature appears to be present in Cambarus. The lateral position of the second maxillae is correlated with the respiratory function of the scaphognathites, which will be described in the section on the respiratory system.
The First Maxillipeds— The first maxillipeds (fig. 44 F) somewhat resemble the second maxillae (D) in that each bears a pair of endite lobes and has a large coxal epipodite (F, Eppd), but they differ from the maxillae in having both an endopodite and an exopodite. The endopodite is a small, simple lobe (Endpd), but the exopodite (Expd) is a typically developed outer ramus consisting of a long basal segment and a terminal flagellum. The basal part of the maxilliped is evidently a combination of the coxopodite and the basipodite, that is, a protopodite, and the two endites apparently are to be referred one to the coxopodite, the other to the basipodite, as in the maxilla. The epipodite is of special interest; it is a long, broad, troughlike structure, concave dorsally, that extends posteriorly from the base of the appendage into the upper part of the anterior end of the gill chamber. The posterior lobe of the maxillary scaphognathite lies snugly in the dorsal concavity of the maxilliped epipodite (fig. 48 B), and the latter thus forms a conduit from the gill chamber by which water may be conducted into the pump chamber over the scaphognathite (A).
The Second Maxillipeds— The second maxillipeds (fig. 44 G) retain more of the form of legs than do any of the appendages before them. Functionally, however, they are a part of the feeding apparatus and project horizontally forward beneath the preceding mouth parts. Each appendage is a biramous limb with seven segments in the main shaft, though the basipodite and ischiopodite are united with each other. The large coxopodite (Cxpd) carries a bilamellate epipodite (Eppd) that bears the first coxal gill, or podobranchia (pBrn), and projects upward and posteriorly into the anterior end of the gill chamber beneath the epipodite of the first maxilliped. Associated with the coxa is also a single arthrobranchia (aBrn) arising on the articular membrane at the base of the coxa. The relatively short, five-segmented endopodite (Endpd) is armed distally with strong spines that probably serve for grasping food introduced between the third maxillipeds. The long, slender exopodite (Expd) is of the usual form, consisting of an elongate basal segment and a distal flagellum. Endites are absent.
The Third Maxillipeds— The maxillipeds of the third pair (fig. 44 H) are the most leglike in form of any of the appendages anterior to the chelipeds, but their principal function is concerned with feeding. Each appendage is a long, seven-segmented shaft, with a relatively small exopodite (Expd) arising from the basipodite (Bspd). The coxa carries a gill-bearing epipodite, and associated with each third maxilliped are two arthrobranchiae (not shown in the figure). On the anterior surface of the coxa arises a tuft of long, tangled filaments (cxf) such as occur also on the coxae of the first four pairs of pereiopods. The endopodites of the third maxillipeds project forward horizontally beneath the other mouth parts (fig. 45 A), and the inner surfaces of the long ischiopodites are armed with combs of strong teeth above fringes of long hairs directed mesally. The mouth of the crayfish lies above these combs of the third maxillipeds, and the food is introduced between them by the small chelae of the second and third pereiopods.
The First Pereiopods, or Chelipeds— The first pereiopods of the crayfish are the huge pincer-bearing chelipeds (fig. 40, Chpd) following the third maxillipeds (fig. 45 A). They have the same segmentation as the legs, except for a union of the ischiopodites with the basipodites. The forceps, or chela, of each appendage (B) consists of the greatly enlarged propodite (Propd), with a strong clawlike lateral extension that forms the fixed finger, and of the dactylopodite (Dactpd), which is articulated on the propodite at the base of the fixed finger and constitutes the movable finger or effective part of the instrument. The coxa of each cheliped bears a podobranchia attached on an epipodite, and associated with it are two arthrobranchiae. The part of the limb beyond the coxa turns up and down on a horizontal hinge with the coxa, but the meropodite moves laterally on the ischiopodite. The “knee” joint between the meropodite and the carpopodite flexes as usual in a vertical plane, but at the next two joints the movement is horizontal.
Fig. 45. Crustacea—Decapoda. Cambarus longulus Girard. The thoracic appendages.
A, third maxillipeds, chelipeds, and first two pairs of legs, male, ventral. B, left chela of female, with muscle apodemes. C, lengthwise section of base of chela, showing adductor apodeme and muscle fibers. D, left cheliped, flexed to full extent. E, third left leg and pleural attachment, posterior. F, base of fourth left leg and pleuron of last thoracic segment. G, dactylopodite and its muscles in propodite. H, ventral part of last thoracic segment of male, posterior, showing penes on coxopodites. I, base of left second leg of female, with genital aperture (Gpr) on coxopodite.
For explanation of lettering see pages 190–192.
When the chelipeds are extended anteriorly (figs. 40, 45 A), the broad chelae lie almost horizontally, or with their dorsal surfaces sloping downward to the sides, and the movable fingers are mesal. Inasmuch as the movable finger of the chela is the dactylopodite of the appendage, which ordinarily moves in a vertical plane, and since the fingers of the small chelae on the next two pairs of legs are dorsal (fig. 45 A), it is probable that the chelae of the chelipeds have become horizontal by a twisting of the appendages. The dactylopodite of the chela, therefore, is hinged on the “hand,” or propodite, by dorsal and ventral articulations (B) so that it moves in a transverse plane; the “hand” is similarly movable on the carpopodite. When the upper or lower wall of the propodite is removed, the cavity of the segment is seen to be filled with a great mass of oblique muscle fibers attached on the dorsal and ventral walls. The fibers in the mesal part of the segment converge to their insertions on a slender apodeme (B, abAp) attached to the inner basal angle of the movable finger; the much more numerous fibers in the lateral part are inserted on both sides of a large, horizontal apodemal plate (adAp) attached on the outer basal angle of the finger. The two sets of fibers are respectively the abductor and adductor muscles of the dactylopodite. As seen in a vertical section of the propodite (C), the adductor fibers go at an angle of about 45 degrees from their origins to their insertions, and it might therefore seem that they can exert but a very limited pull on the base of the movable finger. It will be found, however, that in order to close the widely opened pincers, only a relatively small movement of the adductor apodeme is necessary to bring the points of the claws together.
The chelae of the chelipeds are the principal organs of the crayfish for grasping and holding prey, but when either appendage is strongly flexed at the knee joint and the proximal part is bent back on the coxa as far as it will go (fig. 45 D), the large chelae are still far from the mouth; however, they come within easy reach of the small pincers on the first two pairs of legs (A, 1L, 2L).
The Second and Third Pereiopods— These two pairs of appendages, which functionally are the first and second legs, have the form of simple ambulatory limbs, except that each bears a small chela at the end of the long slender propodite (fig. 45 A, 1L, 2L). With the miniature chelae on these slender, flexible legs the crayfish is able to take food from the chelae of the chelipeds and insert it between the toothed inner edges of the meropodites of the third maxillipeds (A, 3Mxpd). The food is then probably taken over by the strongly spined apical segments of the second maxillipeds (fig. 44 G), from which it must finally be transferred to the mandibles by the fringed endites of the first maxillipeds (F) and the two pairs of maxillae (C, D). All the appendages, therefore, in the series from the mandibles to the second legs, inclusive, are in one way or another implicated in the function of feeding.
The coxae of the third pereiopods, or second legs, of the female give exit to the genital ducts. Each genital aperture is a crescentshaped slit (fig. 45 I, Gpr) on the mesal posterior part of the ventral surface of the coxa but is closed by a large operculum (Opl) that looks like an oval disc set into the coxal wall. The position of the two orifices is such that the discharged eggs are readily received into the channel of the sternum, where they can flow back to the annulus ventralis. In the male of Cambarus and some other genera a small, curved spine on the ventral side of the ischiopodite of each third pereiopod (fig. 45 A, j) serves as a clasping organ during mating, it being hooked over the joint between the second and third segments of the corresponding leg of the female.
The Fourth Pereiopods— This pair of appendages will serve best for a study of the structure of a typical ambulatory limb (fig. 45 E). The large coxopodite is articulated dorsolaterally (a) on the pleuron (Pl) and ventromesally (b) on the sternum of its segment. Since the pleural articulation is anterior and the sternal articulation is posterior, the axis of rotation of the coxa on the body is oblique in two directions. The movements of the coxa, therefore, turn the extended leg forward and mesally in promotion, and posteriorly and outward in remotion. Between the coxa and the basipodite the hinge is horizontal, and the leg is simply elevated or depressed at this joint. The hinge lines at the basi-ischial joint and at the ischiomeral joint are oblique in a vertical plane and permit only a limited sidewise movement. The rigidity of these two joints in the vertical plane allows the levator and depressor muscles of the basipodite to lift or lower the body on the distal part of the leg. The “knee” joint of the leg is the prominent bend between the meropodite and the carpopodite, and the movement of the carpopodite is restricted to the vertical plane. The propodite again is movable transversely on the carpopodite, and finally the simple, clawlike dactylopodite turns up and down on the end of the propodite. The many-jointed arthropod limb, therefore, while it has no twisting motion such as that of the human arm, can perform almost any kind of movement by reason of the different actions at the joints between its seven segments. The leg muscles, which are fully described in Astacus by Schmidt (1915), are appropriate to the specific movements of the limb segments as determined by the intersegmental articulations. The muscles of the dactylopodite of the Crustacea arise entirely in the propodite (fig. 45 G).
The coxa of the fourth pereiopod, as the coxae of the preceding legs, carries on its upper end a gill-bearing epipodite (removed in fig. 45 E), and two arthrobranchiae arise from the articular membrane above it (also removed in the figure). On the anterior face of the coxa is a group of long slender filaments, such as those noted on the third maxillipeds, which are present also on all the legs but the last. It is probable that these filaments act as strainers guarding the entrances to the gill chambers between the mesal ends of the coxae. From the pleurocoxal membrane behind the fourth pereiopod arises a small pendent lobe bearing a brush of long hairs (E, F, g); the nature and function of this organ is not known.
The Fifth Pereiopods— The legs of the last pair are similar to the preceding legs, differing from them principally in that the clawlike dactylopodites are turned forward instead of downward (fig. 40). There are no gills associated with these legs. The coxopodite articulates laterally in a notch on the lower edge of the pleural plate of the last thoracic segment (fig. 45 F, a) and mesally on the sternum (H, b). The two articulations are approximately in the same transverse plane, so that the movements of these legs on the body are more directly anterior and posterior than are those of the other legs. In the female the mesal surfaces of the coxopodites of the last legs are evenly rounded; in the male they are produced into conical projections terminating in small, soft, protractile papillae (fig. 45 H, Pen) that contain the openings of the male genital ducts.
The Pleopods— Each of the abdominal segments carries a pair of appendages, but they are not all alike in either sex, and the first two pairs in the male are different from those of the female. Though the term pleopod signifies a “swimming leg,” the abdominal appendages of the crayfish serve various purposes; the first five pairs in the female are used for carrying the eggs and the young, the first two pairs in the male are accessory genital organs for sperm transfer, while the last pair in each sex, distinguished as the uropods, are the principal swimming organs.
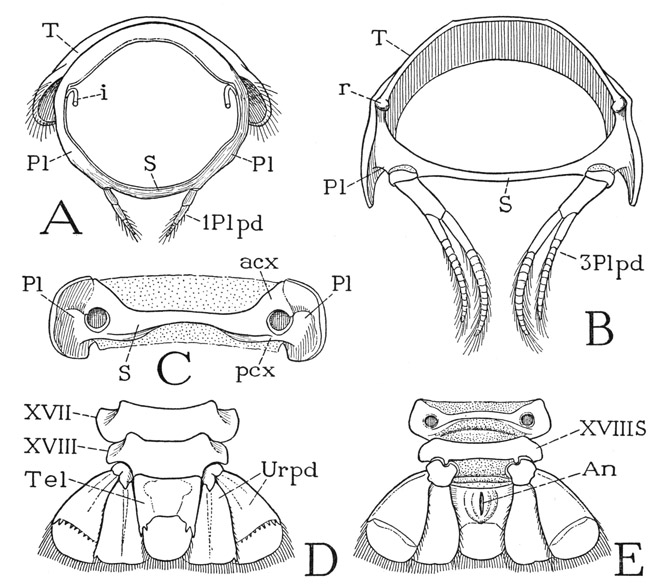
Fig. 46. Crustacea—Decapoda. Cambarus longulus Girard. The abdomen and the pleopods.
A, first abdominal segment of female, anterior. B, third abdominal segment of female, anterior. C, ventral surface of third abdominal segment of female, pleopods removed. D, last two abdominal segments, with telson and uropods, dorsal. E, same, ventral.
For explanation of lettering see pages 190–192.
The first pair of pleopods of the female (fig. 46 A, 1Plpd) are small, simple appendages situated relatively close together on the undersurface of the abdomen. Each consists of two elongate basal segments and a short terminal flagellum. The following four pleopods of the female are much larger biramous appendages and arise near the lateral extremities of the ventral arc of the segment (B). The stalk of each of these pleopods consists of a long basipodite and a small incomplete basal ring apparently representing the coxopodite. The endopodite and the shorter exopodite have each an elongate basal segment and an articulated distal flagellum. The articles of the flagellum are not to be regarded as segments, since they are not interconnected by muscles, but, as shown by Schmidt (1915), a muscle enters the base of the flagellum and branches to each of the subdivisions. The same is true of the flagella of the maxillipeds, but not of the antennae.
The uropods (fig. 46 D, E) have the same form in both sexes. Each uropod is a biramous appendage in which the two rami are broad, flat, triangular lobes supported on a small basal segment, which is articulated on a pivotlike process of the posterior margin of the sixth abdominal sternum. The exopodite has a line of flexure across the middle armed dorsally (D) with small spines, and each lobe is fringed with long hairs. The uropods and the telson constitute the “tail fan,” and are provided with numerous muscles, while the abdomen itself is filled with a great mass of muscles. In using the tail fan in swimming, the crayfish gives a powerful stroke of the abdomen downward and forward, by which it violently propels itself backward.
In the male crayfish the pleopods of the third, fourth, and fifth pairs and the uropods are like those of the female; the first two pairs, being modified and used for reproductive purposes, may be termed gonopods. Ordinarily the gonopods project horizontally forward in the sternal groove beneath the thorax, so that in describing them the anterior surfaces may be said to be dorsal, and the posterior surfaces ventral. Because those of the second pair are the less modified of the two, they will be described first.
By comparison with a pair of typical pleopods, the second male gonopods (fig. 47 B) are seen to be a simple modification of a biramous abdominal limb. Each has a strong, two-segmented basal stalk bearing an exopodite and an endopodite. The exopodite (Expd) is of the usual flagellar structure, but the much larger endopodite (Endpd) has a long, thick basal segment that is produced at its distal end into a small lobe (n) turned dorsally, and it bears a short terminal flagellum. The only special feature, then, of this gonopod is the lobe on the endopodite. The lobe stands vertically on the dorsal edge of the endopodite (F, n); it is triangular in shape, with the anterior angle produced in a long, free point, and the oblique anterior margin is rolled laterally in a wide flange.
Fig. 47. Crustacea—Decapoda. The male gonopods.
A, Orconectes virilis (Hagen), first gonopods, ventral. B, same, second gonopods, ventral. C, Orconectes limosus (Raf.), first left gonopod, posteromesal (from Andrews, 1911). D, same, first left gonopod, ventral (from Andrews, 1911). E, Cambarus longulus Girard, first right gonopod, mesal. F, same, distal part of second left gonopod, mesal. G, same, first right gonopod, dorsal. H, same gonopod, lateral. I, same gonopod ventral. J, Orconectes limosus (Raf.), proximal section of first gonopod and lobe (F, n) of second gonopod (from Andrews, 1911). K, same, section through distal part of gonopod (from Andrews, 1911).
For explanation of lettering see pages 190–192.
The first gonopods of a mature male are strong, unsegmented shafts arising close together on the ventral arc of the first abdominal segment (fig. 47 A), but their form is so different in different species that only a few individual examples can be given here. A relatively simple structure of the first gonopods is seen in Orconectes limosus (C, D), which has been fully described and illustrated by Andrews (1911). At about the middle of the ventral (posterior) surface of each appendage there begins on the mesal margin a deep groove (D, gr) that curves laterally, then runs forward, going beneath the base of a long tapering subapical lobe (m) that overhangs it laterally, and finally runs out on the sharp apex of the main shaft (Can), termed the cannula by Andrews. At its proximal end the groove is widely open by an aperture on the mesal surface that can be seen on turning the gonopod laterally (C, o).
The first gonopods of Orconectes virilis (fig. 47 A) have essentially the same structure as those of O. limosus, but the accessory lobes (m) are longer and the cannulae (Can) are drawn out into long, tapering processes. In Cambarus longulus, on the other hand (E, G, H, I), the distal parts of the appendages are short; the accessory lobe (m) is flat and triangular; the cannula (Can) is broad, rounded, and decurved. The seminal groove (gr) begins at about the middle of the mesal surface (E, o), curves laterally past the base of the accessory lobe (I, m), and then turns downward on the mesal surface of the broad cannula (E, I, Can). The second gonopods of C. longulus resemble those of Orconectes virilis (B).
During conjugation of the male and female crayfish, as described by Andrews (1911), the protruded genital papilla on either coxa of the last thoracic legs of the male (fig. 45 H, Pen) extends over the dorsal side of the corresponding first gonopod, and its decurved extremity is pressed into the aperture of the groove on the mesal surface. The triangular lobe of the second gonopod (fig. 47 B, F, n) on the same side is now pressed against the genital papilla and held there by the insertion of its marginal flange into the groove of the first gonopod (J, K) thus giving a watertight passage for the sperm from the papilla into the groove. Andrews notes that the lobe of the second gonopod may be moved back and forth in the groove of the first, but he doubts that this movement has anything to do with the propulsion of sperm through the canal. It is the function of the first gonopods, however, to discharge the sperm from the apical cannulae into the aperture of the annulus ventralis of the female. In order to do this, the gonopods have to be lowered at an angle of about 45 degrees from the horizontal, and to hold them in this position, it is observed by Andrews, one or the other of the last thoracic legs is thrust transversely between the body and the gonopods.
The great variation in form of the first gonopods of cambarine crayfishes affords the best characters by which taxonomists are able to determine species. Numerous illustrations of their form and a review of their nomenclature for taxonomic purposes are given in several papers by Hobbs (1940, 1942, 1945).
The Respiratory System
The structures concerned with the exchange of respiratory gases between the water and the blood and the maintenance of a water current are all external in the crayfish. They include the gills, or branchiae, the branchial chambers, and the respiratory pump.
The gills of the crayfish are entirely enclosed in the branchial chambers beneath the descending lateral folds, or branchiostegites, of the carapace. In all there are 17 gills crowded into each chamber. Six of them in an outermost row arise on the coxae of the appendages, and hence are termed podobranchiae; the other eleven arise from the articular membranes above the coxae, and are distinguished as arthrobranchiae. In some decapods there are also pleurobranchiae arising from the pleural walls of the gill chambers. The podobranchiae of the crayfish pertain to the second and third maxillipeds and the first four pereiopods. The arthrobranchiae arise from the articular membranes of these same appendages, but there are two of them corresponding to each podobranchia, one anterior, the other posterior, except on the second maxilliped, which has only a posterior arthrobranchia. The fifth pereiopods carry no gills of either kind. The podobranchiae are larger than the arthrobranchiae and increase in size from before backward as the height of the branchial chamber increases; they slope first upward and posteriorly from the coxae, each partly overlapping the one behind, but the upper ends are curved forward, and the forward curvature becomes successively greater to the fifth gill.
Each gill superficially appears to be a mass of soft filaments directed away from the base of the organ (fig. 48 C). The branchial structure is more evident when most of the filaments are removed. It is then seen in a typical podobranchia, such as the one on the second leg (D), that the filaments arise entirely from a long, curved, axial shaft ending in a slender apical process, and that the branchial shaft is attached for much of its length to the outer side of a membranous, trough-shaped coxal epipodite (Eppd), which expands distally into two broad, thin, vertical lamellae with thickened margins and lengthwise corrugations. The structure of the epipodite with its corrugated lamellae is best seen when the gill is turned over, exposing the mesal surface (E), or in a dorsal view (F). A cross section (G) along the line x–y in F shows the trough shape of the epipodite (Eppd), which becomes accentuated distally, and the attachment of the tubular branchial shaft to the outer side of the epipodite trough. In the lobster, Homarus, the podobranchiae arise from the bases of the epipodites and project freely from them. By comparison with Anaspides (fig. 37) it will be seen that in the latter it is the epipodites themselves that serve as gills. The podobranchiae of the fourth pereiopods (fig. 48 H) in the crayfish have the same structure as those of the preceding segments, but the epipodite (Eppd) is much reduced in size, having an expanded base from which only a slender tapering arm runs out in conjunction with the proximal half of the branchial shaft. The arthrobranchiae are simple plumose gills (I) in which the axial shaft arises directly from the articular membrane above the coxa.
The branchial chambers have been sufficiently described in connection with the thoracic skeleton. By comparison with the thorax of Anaspides (fig. 41 A), in which the gills are completely exposed, a cross section of the decapod thorax (D) suggests that the branchiostegites are folds of the tergum (tf) that have grown down from the back to form protective covers for the gills. The outer walls of the branchial chambers are the inner lamellae of the tergal folds, the sclerotic inner walls are the united pleura of the segments involved.
Each branchial chamber extends from the second maxilliped segment to the end of the carapace. In the maxilliped region the sclerotic pleural wall of each chamber (fig. 41 D, Pl) turns abruptly downward and becomes horizontal as it continues forward in the maxillary region (C, Pl), where it forms the broad pleural bridge connecting the maxillary foramen with the base of the inner lamella of the carapace fold (fig. 42 A, mxB). The branchial passage is thus continued anteriorly into a shallower chamber (fig. 48 A, PC), also covered laterally by the carapace fold but shut in ventrally by the large epipodite of the first maxilliped (Eppd) and the scaphognathite of the second maxilla (Scpg). This anterior part of the respiratory passage constitutes the branchial pump chamber (PC). The horizontal scaphognathite lies beneath the maxillary bridge, and its anterior end underlaps the body of the mandible.
Fig. 48. Crustacea—Decapoda. The respiratory system. A–I, Cambarus longulus Girard.
A, diagram of the respiratory pumping apparatus. B, scaphognathite of right second maxilla in trough of epipodite of first maxilliped, dorsal. C, podobranchia of left second leg, lateral. D, same gill, partly denuded of filaments, exposing the supporting epipodite. E, same as D, mesal surface. F, podobranchia of second leg, dorsal. G, section of gill on line x–y in F. H, podobranchia of third leg. I, an arthrobranchia of second-leg segment, left, lateral. J, diagrammatic cross section of a gill of Astacus, showing internal circulatory system (from figures and descriptions by Bock, 1925).
For explanation of lettering see pages 190–192.
We have already noted how the posterior lobe of the scaphognathite rests in the dorsal concavity of the large epipodite of the first maxilliped (fig. 48 B). Posteriorly the epipodite curves upward and forms a troughlike projection into the upper part of the branchial chamber above the first gill (A). The vibratory scaphognathites are the active agents of the respiratory pumping apparatus. If each scaphognathite vibrates in such a manner that its posterior lobe is depressed while the anterior lobe turns up and closes the exit from the pump chamber and then reverses itself on the next stroke, a posterior inhalent force will alternate with an anterior exhalent force. It is evident, then, that water entering the branchial chambers from below and behind the carapace will be drawn forward between and over the gills, finally to be collected in the epipodite troughs of the first maxillipeds and by them delivered into the pump chambers over the scaphognathites. Anteriorly the water is discharged from the pump chambers beneath the antennae.
The true respiration of the crayfish is the exchange of gases between the blood inside the gills and the water in the gill chambers, effected through the walls of the gill filaments. The circulation of the blood within the gills is more difficult to understand than the circulation of water in the branchial chambers, but a detailed account of the inner structure of the gills and a convincing explanation of the course of the blood through them have been given for Astacus by Bock (1925), who shows that the circulatory system within the gills is more complex than had previously been supposed.
The blood, after circulating through the body, collects in the ventral sinus of the thorax and abdomen. In the thorax it is conducted from the sinus into the gills through afferent branchial veins; after circulating through the gills the oxygenated blood is returned to the body by way of efferent branchial veins, from which it is finally conveyed to the heart in the branchiocardiac veins. Within the gill the blood flows distally through an afferent canal (fig. 48 J, afc) and returns through an efferent canal (efc). The afferent canal follows the outer side of the gill shaft, the efferent canal runs along the inner side; these two canals are separated by a common wall, or septum, of connective tissue. It is shown by Bock (1925), however, that a third canal, or sinus, which he calls the mantle canal (mnc), plays an essential part in the circulatory system of the gill. The mantle canal is a space of the gill lumen surrounding the afferent canal on three sides but interrupted where it comes against the walls of the efferent canal.
The lumen of each gill filament is divided into two channels by a lengthwise connective tissue partition, one accommodating the inflowing blood, the other the outflowing blood, the two channels being connected in the apex of the filament. In the lateral filaments of the gill the afferent channels come directly from the afferent canal of the shaft (fig. 48 J, as indicated by the arrows), but the efferent channel of the filament discharges into the outlying mantle canal (mnc). In the filaments adjoining the efferent canal of the shaft (efc), on the other hand, the afferent channels come from the mantle canal, and the efferent channels open directly into the efferent canal of the shaft. In order to get from the afferent canal into the efferent canal of the shaft, therefore, according to Bock’s account, the blood has to circulate through two sets of filaments and is thus twice subjected to oxygenation.
AN ISOPOD, LIGYDA
The crustacean order Isopoda includes a large number of species, most of which are marine, but some live in fresh water, and others are terrestrial. The order includes eight suborders, but the most familiar isopods are those of the suborder Oniscoidea, known as woodlice, sowbugs, pillbugs, and slaters, which are mostly terrestrial, inhabiting moist places under logs and stones, though members of the family Ligiidae live fully exposed on the surfaces of rocks along the ocean shore. One of the ligiids, Ligyda exotica Roux, will be the principal isopod subject of the present chapter; its structure is fairly representative of the Oniscoidea, and in a broad way of that of the isopods in general, but some isopods are parasites on other crustaceans and on fish and have undergone structural and developmental changes in adaptation to their parasitic habits.
Various anatomical features relate the Isopoda to the Amphipoda, the familiar members of the latter group being the “sandfleas” found in rubbish along ocean beaches. In particular, the head in these two orders of malacostracan Crustacea is a definite cephalic capsule, usually separated from the body by a short membranous neck. The head carries the eyes, both pairs of antennae, the mandibles, the first and second maxillae, and at least one pair of maxillipeds; it therefore includes the protocephalon, the three gnathal segments, and the first thoracic segment, all consolidated in a craniumlike tagma that often has a striking resemblance to the head of an orthopteroid insect. The body is divided into a thorax and an abdomen between the same two segments as in the other Malacostraca, but there is no thoracic carapace. The thorax is composed of seven free postcephalic segments, except in some isopods in which a second maxilliped segment enters into the composition of the head.
Carcinologists include the isopods and amphipods together with the Mysidacea, Cumacea, and Tanaidacea in a superorder Peracarida, characterized in part by the presence in the female of most species of large, thin mesal lobes of the coxae, termed oostegites, that come together beneath the thorax to form a brood chamber in which the eggs and young are carried. The head of the tanaidaceans resembles that of the isopods and amphipods, but in the other two peracaridan orders the head is a typical protocephalon, and the gnathal segments are combined with the anterior thoracic segments under a common carapace.
Ligyda exotica (fig. 49 A) is an isopod of the ocean shore, widely distributed in the tropical and warm temperate regions of both hemispheres. On the Atlantic coast of America it occurs from North Carolina to Brazil, and on the Pacific coast from California to Chile. It is often seen in great numbers on flat surfaces of rocks outcropping along the beach. When approached, the animals move swiftly away, but they do not take to the water. The body of Ligyda exotica is elongate oval, about an inch in length, and of a dull gray color. The head bears a pair of long second antennae, and between them a pair of diminutive antennules. Laterally on the top of the head are two large compound eyes. The back plates of the body segments are produced on each side into broad lobes over the bases of the appendages. Several pairs of anterior legs are directed forward, the others backward. From the rear end of the abdomen project two long, slender biramous uropods.
The Head and the Mouth Parts
The head of Ligyda is triangular in facial aspect (fig. 49 B), with the labrum (Lm) at the lower angle and the upper angles capped by the bulging compound eyes (E). The second antennae (removed in the figure) arise from large foramina (2antF) below the eyes; between them are the foramina of the minute first antennae. The mandibles (Md) depend from the sides of the head and close behind the labrum. Two lines crossing the face above the antennae are mere external wrinkles, but a deep groove (es) below them, between the bases of the mandibles, forms an internal shelflike ridge and sets off the region of the epistome (Epst) as a specific area of the facial surface of the head bearing the labrum. The epistome of the isopod is thus an exact replica of the clypeus of an insect (fig. 78 A, Clp); on its basal angles are the anterior articulations of the mandibles (fig. 49 B, c) just as in the insects.
On the back of the head (fig. 49 C) is the large, quadrate neck foramen (For), below which are suspended the maxillipeds (Mxpd). The foramen is margined laterally by narrow rims, which are confluent dorsally in a wider flange (VT) distinctly separated by a groove from the dorsal wall of the cranium before it. Ventrally the foramen is closed by the neck membrane, which, however, contains a crossbar from the posterior end of the maxilliped sternum (VS). Since the dorsal flange over the foramen, the marginal rims on the sides, and the sternal bar below appear to be parts of a circle on the posterior part of the head carrying the maxillipeds, the inference can hardly be avoided that they together represent the reduced annulus of the maxilliped segment united dorsally and laterally with the maxillary part of the cranium. On each side of the neck foramen an apodeme (tAp) is inflected from the groove in front of the marginal rim, which evidently is an ingrowth between the tergal regions of the maxillary and the maxilliped segments.
The ventral wall of the head, when exposed by removal of the mouth parts (fig. 49 H), is seen to have a continuous median sclerotization, from which three pairs of lateral arms are given off. The first arms are intermaxillary brachia (imB) since they go between the bases of the first and the second maxillae; the second are postmaxillary brachia (pmB) between the second maxillae and the maxillipeds; the third are postmaxilliped brachia extending transversely behind the bases of the maxillipeds. The ventral skeleton of the head, therefore, includes the united sternal regions of the first maxillary segment (IIIS), the second maxillary segment (IVS), and the maxilliped segment (VS). The slender intermaxillary brachia (imB) extend laterally and posteriorly, and each ends with a loop in a pocket of the head wall above the outer end of the much thicker postmaxillary brachium of the same side. From the angle of the loop is given off a large apodeme (hAp, only the base shown in the figure) that extends forward in the head cavity. The pair of apodemes arising thus posteriorly in the head of Ligyda corresponds with the first sternal apodemes (the “head apodemes”) of Cambarus, though in the decapods the apodemes arise anteriorly from points near the mesal ends of the intermaxillary brachia (fig. 43 A, 1inv).
Fig. 49. Crustacea—Isopoda. Ligyda exotica Roux.
A, entire animal, male, length of body 24 mm. B, head, anterior, antennae removed. C, head and maxillipeds, posterior. D, right first maxilla, ventral. E, right second maxilla, ventral. F, left mandible, anterodorsal. G, hypopharynx, ventral. H, ventral head skeleton with hypopharynx and mesal lobes of first maxillae attached, second maxillae and maxillipeds removed.
For explanation of lettering see pages 190–192.
Anterior to the sternal skeleton arise the paragnaths (fig. 49 H, Pgn), a pair of large, flat, soft lobes, and between them an elongate, conical median lobe (Lin), which may be termed the lingua. The three lobes have a common skeletal base (G), which gives off a strengthening arm into each paragnath and is itself supported on the arms of the bifurcate first maxillary sternum (G, H, IIIS). The paragnaths and the lingua together are highly suggestive of the three-lobed hypopharynx of certain lower insects (fig. 75 E); in entomological terminology the lateral lobes are the superlinguae.
The mandibles of Ligyda are strong biting and masticatory organs. Each jaw (fig. 49 F) has a boxlike base, from which projects a large gnathal lobe differentiated into a toothed incisor process (inc) and a molar process (md) with a flat, oval mesal surface. The molar processes of the opposite jaws are opposed to each other when the jaws are closed; the incisor processes come together behind the labrum. Each mandible is hinged to the edge of the cranium by the lateral margin of its base and is doubly articulated by an anterior condyle (F, c) and a posterior condyle (a) at opposite ends of the hinge. The anterior condyle articulates on the basal angle of the epistome (B, c), the posterior condyle on the subgenal margin of the cranium. The jaw is activated by at least two muscles; one is a large dorsal adductor (F, dad) attached by a wide thick tendon on the mesal margin of the mandibular base, the other is a slender dorsal abductor (dab) attached on a lever arm of the lateral margin of the mandible near the posterior articular condyle. A small ventral adductor from the head apodeme inserted in the cavity of the mandible is perhaps present, but, if so, the writer has not with certainty identified it in preserved specimens, though such a muscle is present in the amphipods.
The isopod jaws, hanging downward from the head and swinging transversely against each other, are clearly more effective as biting organs than are the mandibles of the decapods. The change in the mandibular mechanism is dependent on the entire reconstruction of the head. The close parallelism in the structure of the head and mandibles between the isopods and the pterygote insects with biting and chewing jaws might suggest that the insects originated from isopods, but, as will be shown later, the mandibles of the more primitive insects have a quite different structure and mechanism.
The maxillae of Ligyda are much simplified appendages. Each first maxilla (fig. 49 D) consists of two elongate lobes, united proximally by membrane and arising from a basal sclerite that articulates on the intermaxillary sternal brachium just mesad of the base of the head apodeme (H). The appendages have wide membranous connections with the head, and the mesal lobes are attached by slender rods to the arms of the first maxillary sternum (IIIS). From a study of Ligyda alone it is impossible to identify the parts of the first maxillae, but the lobes are usually regarded as endites of the base. The second maxillae (E) are still more simplified, each consisting of a single broad lobe supported on a basal sclerite that articulates with the postmaxillary brachium.
The maxillipeds of Ligyda are elongate flattened appendages (fig. 49 C, Mxpd). They are attached to the posterior part of the ventral wall of the head (H, mxpdF) behind the postmaxillary brachia (pmB) at the sides of the narrow maxilliped sternum (VS), the posterior end of which is produced into a pair of postmaxilliped brachia that form a transverse bar (C, H, VS) behind the bases of the appendages. Each maxilliped (C) consists of a small basal segment bearing an epipodite and of an elongate distal segment with a movable subapical lobe.
The Thorax and the Legs
The seven segments of the thorax of Ligyda are of similar shape (fig. 49 A); all are free and flexible on each other. The tergal plates are produced on the sides into broad folds (fig. 50 D, tf) projecting over the leg bases. The ventral surfaces of the segments between the legs are membranous except for the presence of weakly developed transverse sternal bars (D, E, G, S). In the female the apertures of the genital ducts are on the venter of the fifth segment (G) behind the narrow sternum; in the male the ducts open through a pair of penes on the seventh segment (H, Pen).
The legs have each six free segments (fig. 50 A, B), but the long basal segment by which each leg is suspended from beneath the tergal fold is the basipodite (Bspd) and not the coxopodite, as at first sight it might appear to be. The coxopodite is reduced to a narrow ring completely fused to the base of the tergal fold above it (E, F, H, Cxpd), but it bears a large condyle on which the basipodite is articulated. The segments of the functional part of the leg (A, B), therefore, are the basipodite, the ischiopodite, the meropodite, the carpopodite, the propodite, and the dactylopodite. The dactylopodite (C) bears an apical claw (Dac), which has been regarded as the dactylopodite itself, but no muscles are attached on this claw, which is merely flexible on the dactylopodite. The basal muscles of the leg arising on the tergum are inserted on the basipodite, and presumably are the primarily tergocoxal muscles that have been transferred to the basipodite with the suppression of the coxopodite as a movable segment of the limb. In the genus Asellus of the isopod suborder Asellota, the coxae, though small, are free and slightly movable segments.
The lateral folds, or lobes, of the thoracic tergal plates in some of the isopods are set off from the median parts of the terga by grooves, and in some forms they are freely flexible on the back plates. This condition has given rise to the idea that the folds are primarily platelike expansions of the coxae themselves articulated on the terga, and that in those forms, such as Ligyda, in which the lobes are continuous with the terga, they have secondarily united with the latter. This interpretation is deduced by Caiman (1909) from a study of the genus Idotea, in which he says it can be shown in a series of species that the coxae expand to form plates that eventually replace the primary folds of the terga. In Idotea baltica (Pallas), however, the coxae are distinct rings on the undersurfaces of the tergal folds, just as they are in Ligyda, and yet the folds are separated by dorsal grooves from the median tergal plates, on which they are slightly flexible. The muscles of the basipodite always arise on the tergal region mesad of the groove setting off the lateral fold, not on the fold itself, as they might be supposed to do if the fold is a part of the coxopodite. Inasmuch as the abdominal terga are produced into lateral lobes like those of the thorax (fig. 49 A), it seems rational to suppose that the thoracic lobes are primarily tergal and that, with the fusion of the coxae on their undersurfaces, the lobes in some cases have become flexible on the back to allow more freedom of movement to the leg. A separation of the lobes from the back plates is even more pronounced in some of the amphipods, in which the lobes unquestionably appear to be “coxal plates.”
Fig. 50. Crustacea—Isopoda. Ligyda exotica Roux. The thorax and the legs.
A, first left leg of male, lateral. B, sixth left leg of male, lateral. C, dactylopodite and dactyl of sixth leg. D, second thoracic segment of female, posterior. E, fifth thoracic segment of female, ventral, appendages removed. F, left coxopodite and oostegite of fourth thoracic segment of female, ventral. G, fifth thoracic segment of female, showing genital ducts and openings, anterior. H, seventh thoracic segment of male, ventral, with genital ducts and penes.
For explanation of lettering see pages 190–192.
In a mature female the lower surface of the thorax, from the first to the fifth segment inclusive, is concealed by a strongly convex covering of large, thin, semitransparent, underlapping lobes arising from the mesal margins of the coxae on each side (fig. 50 D, Ostg). These lobes are the oostegites, so named because they form a deep protective pouch under the body in which the eggs and young are carried. The oostegites underlap each other from behind forward, and those on the right underlap those on the left. On the second, third, and fourth segments the oostegites are large, broad lobes (F), each strengthened by a rib in the anterior part and a thickened posterior margin; they are supported by the long basipodites of the legs (D). The first and fifth pairs are shorter and narrower than the others and lie more transversely (G), so that they close the two ends of the baglike brood pouch.
The Abdomen and the Pleopods
The abdomen of Ligyda is somewhat ovate in outline as seen from above (fig. 49 A) with the larger end forward. The first five tergal plates resemble the thoracic terga, except that the first and second are smaller than the others. The sixth tergum is a quadrate plate produced into an obtuse angle forming the apical point of the body between the bases of the uropods.
The undersurface of the abdomen is covered by two rows of large, flat, soft lobes, which are the exopodites of the first five pairs of pleopods, underlapping each other from before backward like a double series of scales. The lobes enclose above them a branchial chamber beneath the lower surface of the abdomen, which contains the gills borne on the bases of the pleopods. The true ventral wall of the abdomen (fig. 51 A) can be seen only by removal of the pleopods. Each segmental area of the venter except the last is mostly membranous but is bordered anteriorly by a marginal sternal bar, the outer ends of which curve posteriorly and then mesally around the bases of the pleopods and enclose keyholelike foramina from which the pleopods arise. On the third, fourth, and fifth segments the middle of each sternal bar is produced into a large, tapering process directed posteriorly. The sternum of the sixth segment is a broad plate (XVIIIS) between the lateral lobes of the tergum, and the appendages of this segment, the uropods (Urpd), project posteriorly from it. The telson of Ligyda is represented only by a pair of lobes enclosing the anus (An) on the underside of the projecting end of the sixth tergum.
The pleopods of Ligyda and other isopods have little resemblance to those of Anaspides or Cambarus, and they carry the gills. The first five pairs arise anteriorly from the ventral areas of their respective segments, and all but the first have long transverse connections with the body (fig. 51 A), extending from the median sternal processes to the bases of the lateral tergal lobes. The first pleopods (fig. 51 C) differ from the following appendages, but they are alike in the two sexes. Each has a thick, transversely elongate basal part, or protopodite (Prtpd), which bears on its lateral end a small epipodite (Eppd) and, arising from its ventral surface, a large flat lobe regarded as the exopodite; an endopodite is absent. The ventral surface and the outer end of the protopodite is traversed by a deep cleft, the inner end of which turns forward within the protopodite, and on its anterior lamella is attached a transverse row of short muscle fibers, which evidently serve to open the cleft. The function of this structure is unexplained. The second pleopods are different in the two sexes. In the male each appendage (D) has a large, flat exopodite (Expd) like that of the first pleopods, and also an epipodite (Eppd), but in addition to these parts there arises from the protopodite a long, sclerotic, two-segmented, elbowed endopodite (Endpd), which presumably has some copulatory function. The much simpler corresponding pleopod of the female (F) bears, in the position of the male endopodite, a small, dorsal gill lobe (Brn), which is regarded as the endopodite of the appendage, though the interpretation may be questioned. The next three pairs of pleopods, of which the third in the female (G) or the fourth in the male (E) may be taken as an example, are again alike in both sexes and resemble the second pleopod of the female (F), except that the gill lobe (Brn) is much larger and there is no epipodite on the protopodite. Since Ligyda exotica is often found on rocky beaches well back from the “spray zone,” it is not clear how its gills function as respiratory organs in dry air. The uropods of Ligyda are simple biramous appendages (fig. 49 A), each consisting, as already noted, of an elongate basal stalk and two slender rami.
Fig. 51. Crustacea—Isopoda. Ligyda exotica Roux, and Armadillidium sp. The abdomen and pleopods.
A, Ligyda exotica Roux, abdomen of male, ventral, pleopods removed. B, same, fifth abdominal segment of male, ventral. C, same, first left pleopod of male, dorsal. D, same, second left pleopod of male, dorsal. E, same, fourth left pleopod of male, dorsal. F, same, second left pleopod of female, dorsal. G, same, third left pleopod of female, dorsal. H, Armadillidium sp., third left pleopod of male, dorsal. I, same, posterior edge of exopodite of first right pleopod, showing marginal depression with three “spiracles.” J, same, branched lobes of tracheal organ. K, same, first and second pleopods and penis of male, ventral, showing respiratory organs (Tra) in exopodites.
For explanation of lettering see pages 190–192.
The common terrestrial inland isopods, such as Porcellio and Armadillidium, have, in addition to gills, respiratory organs for air breathing contained in the first two pairs of pleopods. These organs appear as conspicuous “white bodies” within the exopodites of the first and second pleopods in both sexes (fig. 51 K, Tra). When dissected from a fresh specimen, the “bodies” are found to be masses of hollow, branching lobes having a soft, granular texture (J). If these organs are truly respiratory in function, as they are supposed to be, the branched lobes are of the nature of primitive tracheae; they open through apertures in a troughlike depression on the posterior margin of the exopodite (I). In the male of Armadillidium each pleopod of the first two pairs (K) has a long endopodite. The next three pairs of pleopods are alike in the two sexes, and resemble the corresponding pleopods of Ligyda; each has a flat gill lobe (H, Brn) arising dorsally from the protopodite. The underlapping exopodites, as in Ligyda, enclose the gills in a branchial chamber beneath the abdomen, but the apertures of the tracheal organs are fully exposed. The gills of these terrestrial isopods are supposed to function in particularly moist places, or when water enters the gill chamber.
Explanation of Lettering on Figures 37–51
a, pleural articulation of coxa.
abAp, abductor apodemc.
Abd, abdomen.
aBrn, arthrobranchia.
acx, antecoxal pleurosternal bridge.
adAp, adductor apodeme.
atc, afferent canal of gill.
An, anus.
Ant, antenna; 1Ant, first antenna, or antennule, 2Ant, second antenna.
antF, antennal foramen.
Ap, apodeme.
b, sternal articulation of coxa.
BC, body cavity, haemocoele.
bcg, branchiocardiac groove.
brC, branchial chamber.
Brn, branchia, gill.
Bspd, basipodite.
c, epistomal articulation of mandible.
Can, cannula.
Ch, chela.
Chpd, cheliped or cheliped foramen.
Cor, cornea.
Cp, carapace:
Crppd, carpopodite.
cvg, “cervical” groove.
cxf, coxal filaments.
Cxpd, Cx, coxopodite.
da, anterior dorsal muscle.
dab, dorsal abductor muscle.
Dac, dactyl, claw of dactylopodite.
Dactpd, dactylopodite.
dad, dorsal adductor muscle.
Dct, duct.
Dej, ductus ejaculatorius.
df, dorsal filament of gill.
dp, posterior dorsal muscle.
e, ocular plate of protocephalon.
E, compound eye.
efe, efferent canal of gill.
Endpd, endopodite.
Endt, endite.
Eppd, epipodite.
Epst, epistome.
es, epistomal sulcus.
esF, foramen of eyestalk.
Expd, exopodite.
ext, extensor muscle.
f, basal sclerite of eyestalk.
fl, flexor muscle.
Fl, flagellum.
For, neck foramen of head.
g, brushlike appendage of thorax of Cambarus.
gnL, gnathal lobe of mandible.
gr, seminal groove.
Gon, gonapophysis.
Gpd, gonopod.
Gpr, gonopore.
h, epistomal hinge of mandible.
hAp, head apodeme (first ventral apodeme).
i, connecting bar between thoracic pleuron and abdomen.
icx, intercoxal pleurosternal brachium.
IIS, metastomal plate, perhaps remnant of mandibular sternum.
imB, intermaxillary brachium.
inc, incisor process of mandible.
inv, point of invagination.
Iscpd, ischiopodite.
I–XVIII, enumeration of segments, beginning with second antennal.
j, spur on second leg of male Cambarus.
k, postantennal wing of epistome.
l, marginal ridge of epistome.
L, leg.
lf, lateral filament of gill.
lF, limb foramen.
Lg, intergnathal ligament.
Lin, lingua.
Lm, labrum.
ltg, laterotergite.
m, accessory lobe of first gonopod.
mcl, muscle.
Md, mandible.
mdB, base of mandible.
mnc, mantle canal of gill.
mol, molar process or area of mandible.
Mrpd, meropodite.
Mth, mouth.
Mx, maxilla; 1Mx, 2Mx, first and second maxillae.
mxB, maxillary bridge (maxillary pleuron).
mxF, maxillary foramen.
Mxpd, maxilliped.
mxpdF, maxilliped foramen.
n, lobe of second gonopod.
npr, nephropore.
o, orifice of seminal groove.
Opl, operculum.
Ostg, oostegite.
p, pivot of distal segment of eyestalk.
pBrn, podobranchia.
PC, pump chamber of respiratory system.
pcx, postcoxal pleurosternal bridge.
Pen, penis.
Pgn, paragnath.
Pl, pleuron.
plAp, pleural apodeme.
Plp, palp.
Plpd, pleopod.
pmB, postmaxillary brachium.
Propd, propodite.
Prpds, pereiopods.
Prtc, protocephalon.
Prtpd, protopodite.
r, articular knob of tergum.
R, rostrum.
S, sternum.
sAp, sternal apodeme.
Scpg, scaphognathite.
Seg, body segment.
slgs, suspensory ligaments.
T, tergum.
tAp, tergal apodeme
Tel, telson.
tf, tergal fold, branchiostegite of decapod thorax.
Tra, tracheal respiratory organ.
Urpd, uropod.
v, ventral muscles of mandible.
Vd, vas deferens.
vmdmcls, ventral mandibular muscles.
VS, sternum of maxilliped segment.
VT, tergum of maxilliped segment.