THE HEXAPODA
THE Hexapoda are the six-legged arthropods commonly known at present as insects; formerly, however, the name “insect” was given to almost any familiar small arthropod, since the bodies of most of them are more or less “insected,” and even today it is difficult to convince some people that spiders, centipedes, and sowbugs are not insects.
The hexapods are divided naturally into three major groups. The first group includes the Protura, Collembola, and Diplura (or Dicellura). These are small wingless forms in which the mandibles and maxillae are enclosed in pockets of the head formed by a union of the labium with the lateral walls of the cranium. From this common character these three orders may be termed the entognathous apterygote hexapods. They have other features, however, that distinguish them from the rest of the hexapods, and some entomologists are reluctant to call them insects. A second hexapod group is the Thysanura, including the well-known families Machilidae and Lepismatidae. The thysanurans also are wingless, but in other respects they are more closely related to the winged insects than to the entognathous apterygotes. The winged insects constitute the third hexapod group, named the Pterygota. The conventional division of the hexapods into Apterygota and Pterygota on the absence or presence of wings is a convenient device for making “keys,” but clearly the absence of wings is not in itself an index of relationship. The wingless entognathous orders are less closely related to the wingless ectognathous Thysanura than are the Thysanura to the winged Pterygota. Even the three entognathous groups themselves differ in many respects from one another and are by no means closely related. Inasmuch as the anatomy of the hexapods is described in various readily accessible textbooks on entomology, only a few examples will be treated here, representing the three major groups given above.
DIPLURA
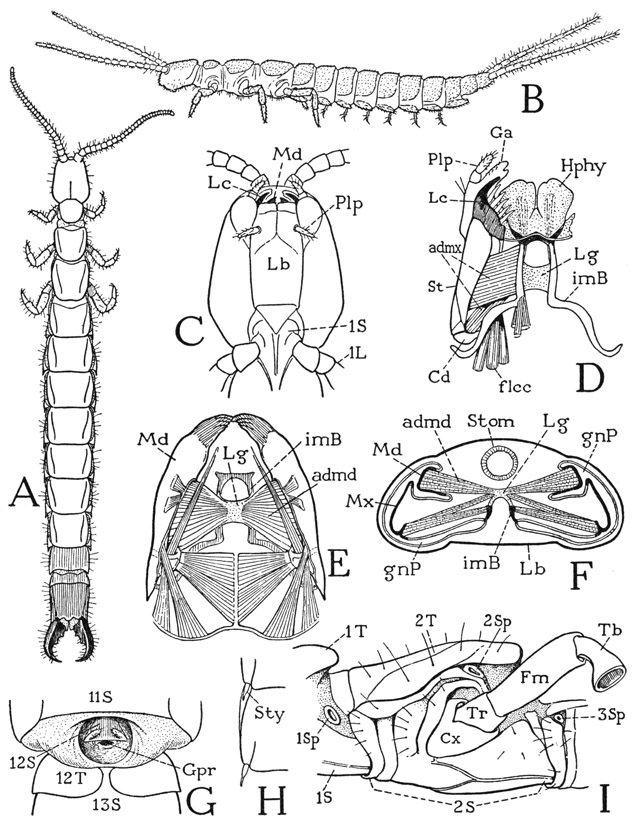
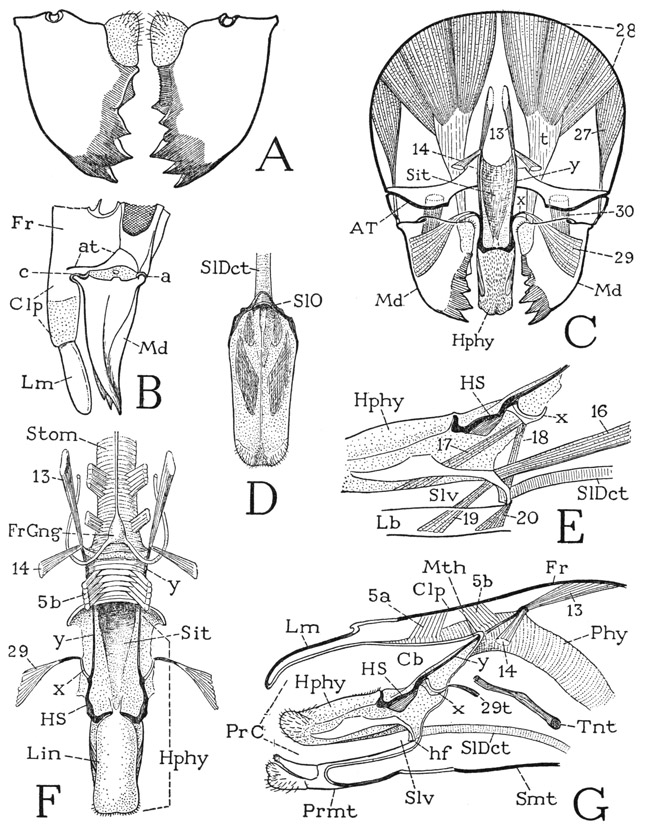
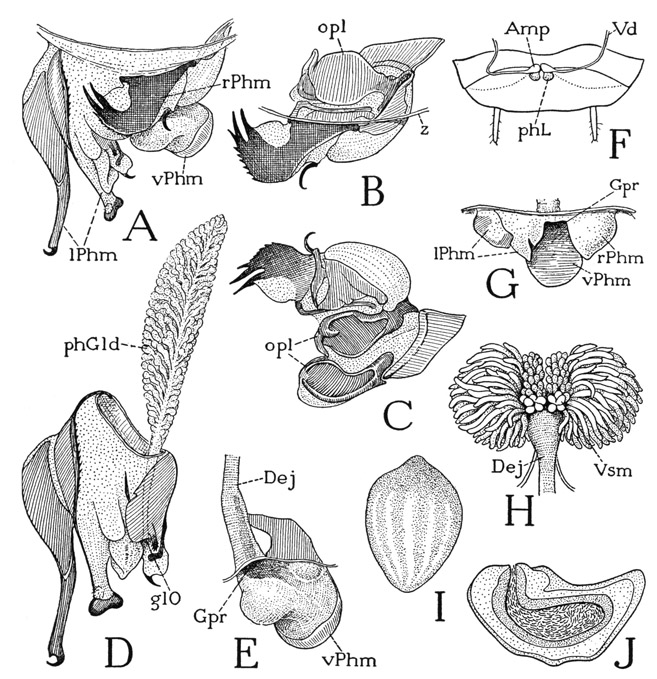
The members of the Diplura, or Dicellura, including the genera Campodea (fig. 74 B), Japyx, Heterojapyx (A), and others, are the most insectlike of the entognathous apterygote hexapods, and some entomologists class them with the Thysanura. The enclosure of the mandibles and maxillae in head pouches, and particular features of the head structure itself, however, leave no doubt that the diplurans belong with the Protura and Collembola and are no more related to the Thysanura than are these two groups.
As an example of the Diplura we may take the relatively huge Heterojapyx gallardi of Australia (fig. 74 A), some individuals of which attain a length of two inches, though the structure will not be essentially different from that of the much smaller, widely distributed species of Japyx. The japygid head is angularly ovate and carries a pair of large antennae, but eyes are absent. The elongate body consists of three thoracic segments bearing the three pairs of legs, and of a ten-segmented abdomen. The last three abdominal segments are strongly sclerotized as compared with those preceding, and the large tenth segment is armed with a powerful forceps. There is no trace of a segment beyond the forceps, the anus being situated in a depression between the bases of the pincers.
On the underside of the head (fig. 74 C) the long median labium, with a pair of small palpi (Plp), is seen to be united on the sides with the ventrally inflected lateral walls of the cranium. The mandibles and maxillae are thus enclosed in a pair of deep lateral pouches above the labium with only their tips (Md, Lc) exposed beyond the latter. The mandibles and maxillae, therefore, are not retracted into the head; they are merely covered below by the labium; their position in the pouches is seen in the cross section at F of the figure (Md, Mx).
The mouth of Heterojapyx is located anteriorly above the free margin of the labium, and just behind it is a large hypopharynx (fig. 74 D, Hphy) composed of two broad superlingual lobes and a small median lingua. A three-lobed hypopharynx is characteristic of the entognathous apterygotes and resembles the three-lobed hypopharynx of the isopod Ligyda (fig. 49 G). From a skeletal support in the base of the hypopharynx of Heterojapyx (fig. 74 D) two long rodlike sclerites (imB) extend posteriorly in the mesal walls of the gnathal pouches (F, imB) and then turn laterally to end behind the bases of the maxillae (D), which are articulated by the cardines (Cd) on them. These posthypopharyngeal sclerites have been regarded as representing the anterior apodemal tentorial arms of Thysanura and Pterygota (see Snodgrass, 1935, p. 118), since they give support to the adductor muscles of the mandibles and maxillae (D, F). However, they are not apodemes, but sclerites of the ventral head wall within the gnathal pouches, present also in Collembola, in which Folsom (1900) has shown that they are formed in the embryo as surface sclerotizations of the sternal wall of the head. Similar rods are present likewise in Protura, but their anterior parts are united in a median sternal bar. In Heterojapyx the anterior parts of the sclerites form internal ridges (F, imB), but their posterior parts are superficial. These sternal sclerites of the head are peculiar to the entognathous apterygotes among the Hexapoda, but they are exact counterparts of the intermaxillary sternal brachia of the amphipod and isopod crustaceans (fig. 49 H, imb), on which are supported the first maxillae.
Fig. 74. Hexapoda—Diplura.
A, Heterojapyx gallardi Tillyard. B, Campodea sp. C, Heterojapyx gallardi Tillyard, head and part of prostemum, ventral. D, same, hypopharynx, intermaxillary brachia, and right maxilla, ventral. E, same, mandibles and their muscles, dorsal. F, same, cross section of head, somewhat diagrammatic. G, same, genital region of male, ventral, exposed by separation of eleventh and twelfth body segments. H, same, right halves of two abdominal sterna, showing styli. I, same, mesothorax and parts of adjoining segments, ventrolateral.
For explanation of lettering see pages 337–339.
In the Japygidae and in Campodea the parallel parts of the two sternal brachia of the head are connected, inside the head, by an arched membranous bridge (fig. 74 D, E, F, Lg), on which are attached adductor muscles of the mandibles and the maxillae. The interbrachial bridge of the Diplura, therefore, evidently represents the intergnathal ligament of the chilopods and diplopods, which in the diplurans has become attached secondarily to the sternal brachia, since the brachia, being intermaxillary in position, cannot belong to the mandibular segment of the head. The entognathous apterygote hexapods have no tentorium corresponding with that of the Thysanura and Pterygota, but in the Collembola the bridge of the sternal brachia is elaborated into a platform for muscle attachments supported on arms of the brachia.
The mandibles of Heterojapyx are slender, elongate organs (fig. 74 E) produced distally into simple, toothed gnathal lobes. The base of each jaw is connected with the mesal wall of the gnathal pouch (F, Md) by a long oval foramen, and the inner end projects into the pouch as a free point (E). The dipluran mandible has no articulation with the cranium either anteriorly or posteriorly, but in Protura and Collembola the inner end of the mandible is connected by a slender rod in the pouch wall with the lateral cranial margin, just as in the chilopods. The principal muscles of the dipluran mandibles (E) include posterior muscles from the cranial wall, attached dorsally and ventrally on the base of the mandible, and adductors (admd) from the interbrachial ligament (Lg) inserted into the cavities of the jaws (F).
The maxillae of Heterojapyx (fig. 74 D) have the structure typical of insect maxillae, except for the reduction of the palpus (Plp). The base of each appendage is divided by a joint into a small proximal cardo (Cd) articulated on the sternal brachium and an elongate stipes (St) bearing two apical lobes, a galea (Ga) and a lacinia (Lc). The adductor muscles (admx) arise partly on the sternal brachia and partly on the connecting ligament (F). The lacinia has a large cranial flexor (D, flcc) characteristic of the insect lacinia. The labium, being united with the lateral walls of the cranium (C, Lb), forms practically a part of the ventral wall of the head.
In the thorax of Heterojapyx each segment has a distinct tergal plate (fig. 74 A, I), but the pleural and sternal parts are united before the bases of the coxae on each side (I). Precoxal folds of the pleuron somewhat resemble the subcoxal sclerites of the chilopods. The dipluran thorax has an unusual feature in the presence of four spiracles, in most insects there being only two. The first spiracle (I, 1Sp) has a lateral position between the prothorax and the mesothorax; the second (2Sp) lies above the coxa of the mesothorax just below the margin of the tergum; the third (3Sp) is on a level with the first in the anterior part of the metathorax; the fourth lies above the metathoracic coxa in the position of the second on the mesothorax. The two more dorsal thoracic spiracles fall in line with the abdominal spiracles. The legs have the six segments characteristic of insect legs (fig. 83 A), but the tarsus is undivided. The pretarsus bears a pair of large lateral claws and a very small median claw; its under part forms an unguitractor plate on which the tendon of the flexor muscle is attached.
The ten segments of the abdomen (fig. 74 A) are alike, except the ninth which is short and the tenth which is longer than the others. On the undersurfaces of the first seven segments are small paired styli arising from the posterior lateral angles of the sternal plates (H). Each stylus is provided with two small muscles. In Campodea (B) the styli are much larger and serve to support the abdomen. The tergum of the short ninth abdominal segment of Heterojapyx, or body segment 12, underlaps the venter (G, 12T), and between it and the sternum of the preceding segment (11S) is a deeply infolded membranous pocket (exposed by separation of the segments in the figure), which contains the genital opening, or gonopore (Gpr), of the male. In front of the gonopore is a small plate bearing a pair of styli, which evidently is the forwardly displaced ninth abdominal sternum (12S). The apical forceps is perhaps serially homologous with the paired styli of the preceding segments. In Campodea (B) the corresponding appendages are long, multiarticulate filaments resembling the antennae. Each jawlike prong of the forceps of Heterojapyx has a large adductor muscle, but no abductor, the pincers remaining open when not muscularly closed. Some interesting observations on the use of the forceps by Japyx are given by Kosareff (1935). Japyx feeds on other small, soft-bodied arthropods such as collembolans and symphylans. As described by Kosareff, the prey is first seized either by the jaws or by the forceps; in either case the abdomen is turned forward over the back and the prey, grasped in the forceps, may be carried around until a suitable place is found for feeding on it; but again, Japyx may devour the prey at once while the latter is held in the forceps.
THYSANURA
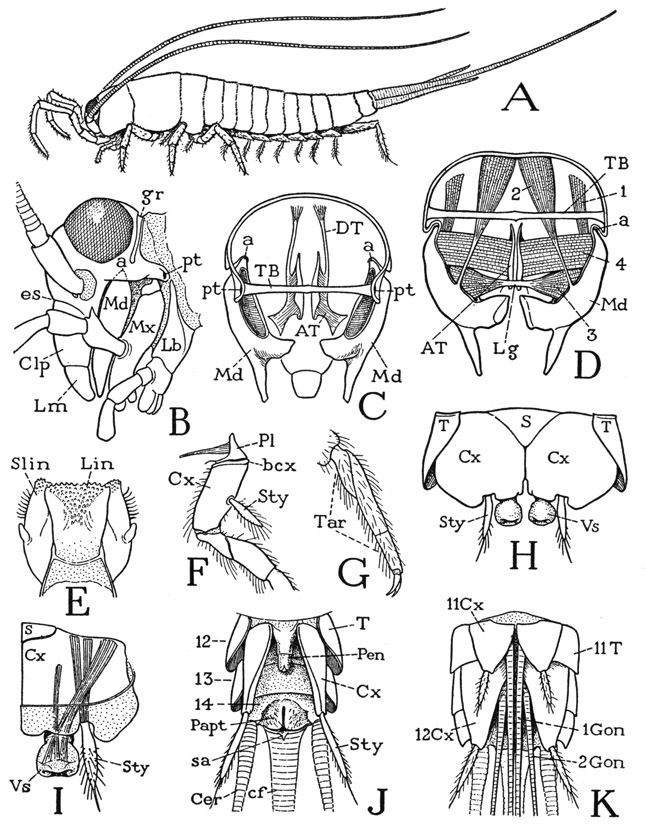
The thysanurans, or bristletails, are small wingless insects of two principal types of structure represented by the families Machilidae (fig. 75 A) and Lepismatidae (fig. 76 A). They derive their name from the fact that the caudal end of the body bears three long, multiarticulate filaments; the middle filament is a prolongation of the tergum of the last abdominal segment; the lateral ones are appendages of the same segment corresponding with the cerci of lower winged insects. The most important features by which the Thysanura differ from the entognathous apterygotes are in the structure of the head, the mandibles, and the antennae. The thysanurans are ectogna-thous, the mandibles and maxillae being fully exposed and the labium a free appendage. The mandibles and maxillae, moreover, are articulated on the lateral margins of the cranium. Sternal sclerites of the head corresponding with the intermaxillary brachia of the Diplura are absent; they are replaced functionally, for muscle attachment, by internal cuticular apodemes that form a true tentorium unquestionably homologous with that of the Pterygota. The antennae consist each of a basal scape and a long, multiarticulate flagellum; their only intrinsic muscles arise in the scape and are inserted on the base of the flagellum (see Imms, 1939). The thysanuran antennae thus have the structure characteristic of the antennae of winged insects and differ radically from the segmented antennae of Diplura, Collembola, and the myriapods. The thysanuran labium has the structure of a generalized pterygote labium. It consists of a large proximal plate, or postmentum (fig. 76 H, Pmt), broadly attached on the head, and of a small, free prementum (Prmt) bearing the palpi and apical lobes. The prementum alone is movable; it has a pair of short muscles arising in the postmentum and other muscles from the tentorium.
The thysanuran body consists of 14 distinct segments, of which three belong to the thorax, but there is no constriction or separation between the thoracic and abdominal regions. Some of the abdominal segments bear styli similar to those of the dipluran Campodea, and in the machilids eversible vesicles are associated with the styli. The styli evidently do not represent abdominal appendages, since Heymons (1897) says the appendage rudiments present in the embryo of Lepisma unite entirely with the sterna, and the styli appear during postembryonic growth. In Ctenolepisma urbana, Lindsay (1940) notes that the ninth-segment styli appear in the fourth instar, those of the eighth segment in the ninth instar of the male and the eleventh of the female. The median caudal filament and the cerci arise from the last abdominal segment, but the muscles of the cerci take their origins in the penultimate segment. The male thysanurans have a simple median penis arising ventrally at the base of the ninth abdominal segment (fig. 75 J, Pen); the females have a long ovipositor formed of two pairs of slender processes borne on the eighth and ninth segments (K, 1Gon, 2Gon).
Important differences between the Machilidae and Lepismatidae are in the mechanism of the mandibles, the structure of the hypopharynx, and the relative development of the tentorium. In these features the machilids are clearly more generalized than the lepismatids, in which the mandibles, the hypopharynx, and, to a lesser extent, the tentorium take on the structure of these parts typical of orthopteroid pterygotes.
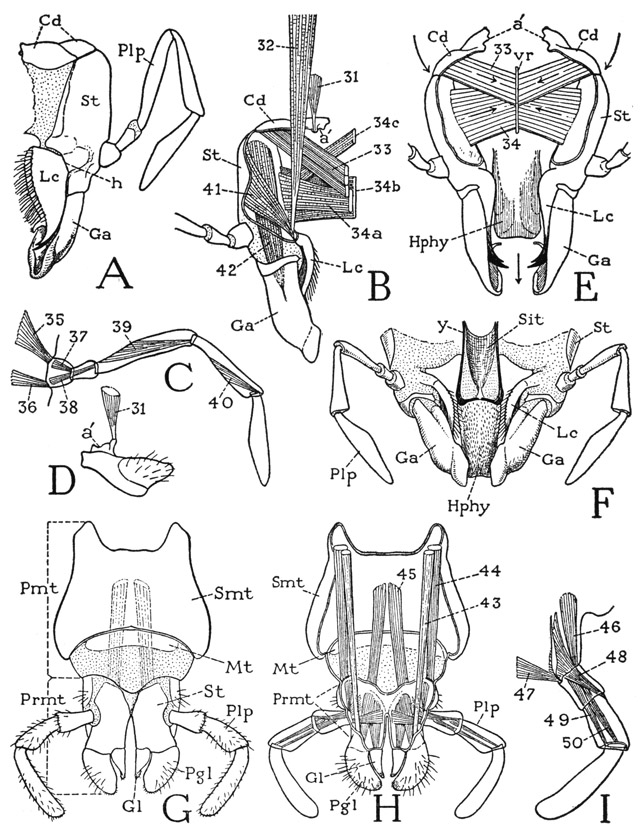
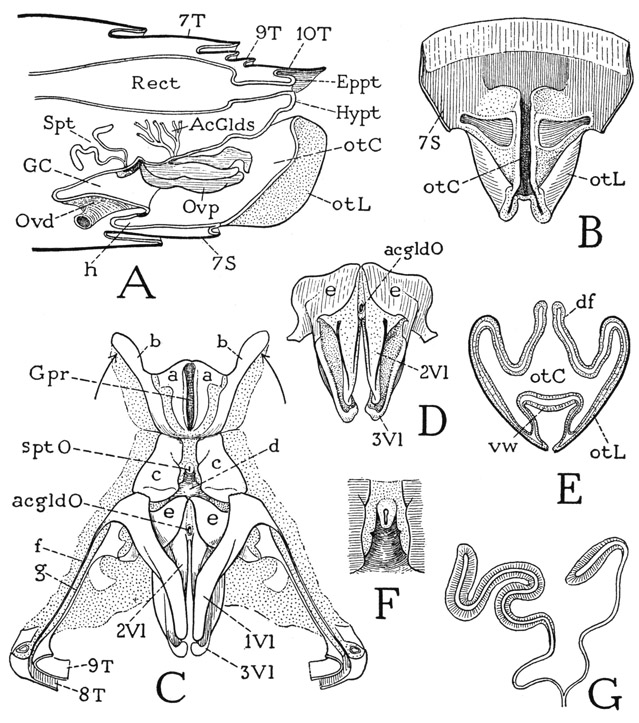
The Machilidae— The machilids (fig. 75 A) can be distinguished from the lepismatids (fig. 76 A) by the more cylindrical body, the shorter cerci, and the greater number of styli, which support the abdomen like a series of small legs. The head (fig. 75 B) is of the hypognathous type of structure with the mouth parts hanging downward from the cranial margin behind a long, lobelike extension of the head below the antennae. The outer surface of the upper, or epistomal, part of the lobe constitutes a distinct clypeus (Clp) set off from the facial region, or frons, above it by a transverse epistomal sulcus (es); the lower part is the labrum (Lm). On the top of the head is a pair of large compound eyes, and behind them a deep, transverse postocular groove (gr). The posterior lateral angles of the cranium are extended posteriorly to give support on each side to the maxilla (Mx) and the labium (Lb). In the end of the cranial extension, between the bases of the two appendages, is a depression, the posterior tentorial pit (pt), from which is invaginated a tentorial bar that goes through the back of the head (C, TB).
The endoskeleton of the machilid head includes three separate tentorial elements; two may be termed the anterior tentorial arms (fig. 75 C, AT), the other is the posterior bar, or tentorial bridge (TB), mentioned above. The anterior arms arise from lateral points of invagination, the anterior tentorial pits, on the ventral side of the head behind the base of the clypeus. Between them is the wide base of the hypopharynx. The arms extend first mesally and then turn posteriorly along the sides of the stomodaeum; each arm gives off a slender dorsal branch (C, DT) attached on the upper wall of the cranium by short muscle fibers. The anterior tentorial arms of the machilids clearly represent the anterior arms of the pterygote tentorium, but, on the other hand, they are so similar to the head apodemes of the chilopods, the diplopods, the pauropods, and the symphylans as to suggest that the anterior head apodemes are homologous structures in all these groups. The posterior tentorial bridge of the machilids (fig. 75 C, TB) is not represented in the myriapods, but in position it corresponds with the posterior arms of the tentorium in the pterygotes.
The machilid hypopharynx is a flattened, three-lobed structure (fig. 75 E), as is that of the Diplura and Symphyla. The median lobe is known as the lingua (Lin); the lateral lobes are termed the superlinguae (Slin) because they arise anterior or dorsal to the lingua.
Fig. 75. Hexapoda—Thysanura. Machilidae.
A, Machilis sp. B, Nesomachilis maoricus Tillyard. C, same, posterior view of head, somewhat diagrammatic, with mandibles in place, showing endoskeleton. D, same, mandibles and their muscles, posterior. E, same, hypopharynx, posterior. F, Machilis sp., base of right metathoracic leg, lateral. G, same, tarsus and pretarsal claws. H, Nesomachilis maoricus Tillyard, ventral surface of a pregenital abdominal segment. I, same, right half of ventral plates of an abdominal segment, with stylus and vesicle, dorsal. J, same, terminal part of male abdomen, ventral. K, Machilis sp., end of female abdomen, with ovipositor, ventral.
For explanation of lettering see pages 337–339.
The mandibles of the Machilidae are elongate jaws (fig. 75 B, Md) hanging from single points of articulation (a) on the cranial margins behind the clypeolabral lobe. The free lower end of each jaw (C, Md) divides into a tapering incisor process and a thick molar process. The mandibular musculature (D) includes four muscles for each jaw, two dorsal in their origin and two ventral. The dorsal muscles are a small anterior rotator (1) and a large posterior rotator (2), both arising on the dorsal wall of the cranium. Of the ventral muscles, which are adductors, one is a broad, flat bundle of fibers (4) arising on the anterior tentorial arm and attached proximally on the mandible; the other (3) is a conical muscle with its fibers spreading into the cavity of the mandible from a median ligament (Lg) on which are attached the fibers of the corresponding muscle of the opposite jaw. The ligament lies just behind the base of the hypopharynx. The machilid jaw is the most primitive mandible found among the insects. A comparison with the mandible of the generalized crustacean Anaspides (fig. 38 E) will show an essential likeness in the structure of the jaws, their articulation, and their musculature, the only difference being the retention of the palpi in Anaspides. The same jaw structure is characteristic also of the branchiopod crustaceans. The musculature of the machilid mandible furnishes the basic pattern of the jaw musculature of Lepismatidae and lower Pterygota.
The thoracic region of the machilid body is distinguished by the large size of its three tergal plates (fig. 75 A) as compared with the abdominal terga. The pleural areas of the thorax are concealed beneath the overhanging lateral edges of the terga; in the mesothorax and metathorax each contains a single triangular pleural sclerite (fig. 75 F, Pl) bearing a large apodeme, situated immediately over the base of the coxa and forming an articular support for the latter. A faint submarginal groove of the coxa sets off a narrow marginal flange, or basicoxite (bcx). On the prothorax, the pleural sclerotization, which has been particularly described by Carpentier (1946), is more complex and somewhat resembles the pleural structure in Lepismatidae (fig. 76 I, J).
The legs are six-segmented, the long tarsus (fig. 75 G, Tar) is divided into three subsegments, and the pretarsus bears only a pair of lateral claws. A special feature of the machilid legs is the presence in most species of a styluslike appendage on the outer side of the coxa (F, Sty) of the second and third legs. These thoracic styli have no muscles.
The machilid abdomen is broadly joined to the thorax (fig. 75 A), and tapers somewhat posteriorly. It consists of 11 segments, but the last segment, which bears the caudal filament and the cerci, is mostly concealed from above by the tenth. On the undersurface of the abdomen are eight pairs of styli pertaining to the second to the ninth segments, inclusive. The ventral surface of each of the first seven segments in the female and the first eight in the male is covered by a pair of large, contiguous lateral plates (H, Cx) and a small median basal plate (S). The lateral plates, except those of the first segment, bear each a stylus (Sty) and are regarded as representing the coxae of primitive abdominal appendages, the median plate (S) being interpreted as the true sternum. In Nesomachilis each of the first seven coxal plates bears also an eversible vesicle (Vs) just mesad of the base of the stylus on the stylus-bearing segments. In some of the machilids several of the abdominal segments may have two pairs of vesicles. The abdominal styli and the vesicles are provided with muscles arising on the supporting coxal plates (I), the styli of the abdomen thus differing from those of the thorax, which have no muscles. On the ninth segment both the coxal plates and the styli are much longer than those of the preceding segments, a sternal plate is absent, and the long, narrow coxal plates are entirely separate (J, Cx). On this segment in the male (J) the median tubular penis (Pen) arises between the bases of the coxal plates. In the female (K) the coxal plates of both the eighth and ninth abdominal segments (body segments 11 and 12) are separated at their bases, and each bears a long, slender genital endite, or gonapophysis (1Gon, 2Gon). The four rodlike gonapophyses are normally closely connected to form a tubular ovipositor. The opening of the oviduct lies between the bases of the first pair of gonapophyses. In the males of some species of Machilis similar but much shorter gonapophyses are present, as in the female, on both the eighth and the ninth abdominal segments, but they are entirely dissociated.
The thirteenth body segment (tenth abdominal) in each sex is a simple annulus (fig. 75 J, 13) without either coxal plates or styli. Following it is the short fourteenth segment (14), which carries the caudal filament (cf) and the cerci (Cer). On its undersurface is a pair of soft valvelike lobes (Papt) enclosing the anus, behind which is a small median lobe (sa), apparently on the base of the caudal filament. The paired lobes probably are the paraprocts of other insects; the postanal lobe (sa) may be the epiproct. The three anal lobes are regarded as representing the telson of more generalized arthropods.
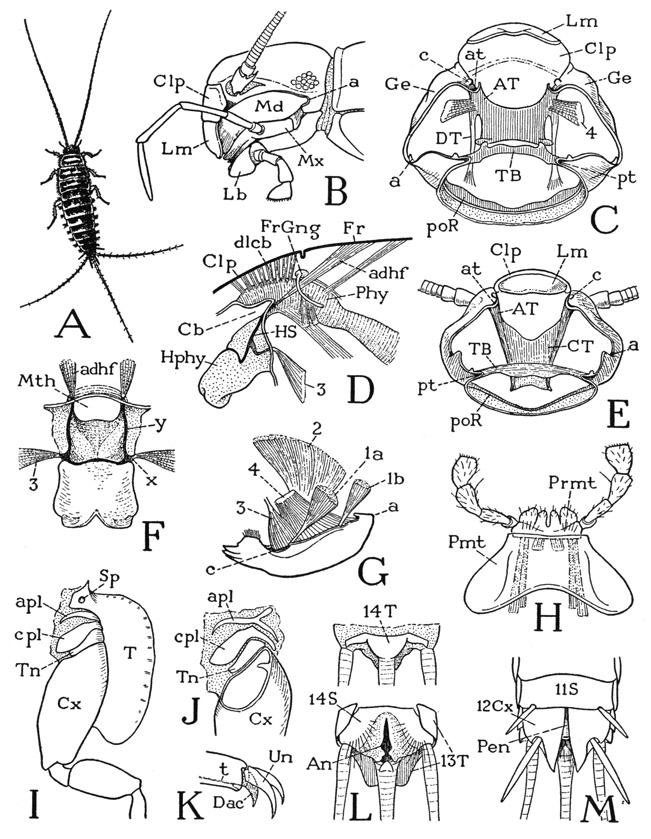
The Lepismatidae— The lepismatids in their general appearance (fig. 76 A) are similar to the machilids, but in several respects they differ from the Machilidae, on the one hand, and, on the other, approach more closely to the Pterygota. The mouth parts are attached on the lateral margins of the cranium (B), but the mandibles (Md) have a long, approximately horizontal connection with the head and are doubly articulated. The primary articulation (a) of each jaw is posterior in a notch of the cranial margin; the secondary anterior articulation is by means of a small condyle on the ventrally inflected angle of the gena below the base of the antenna (C, E, c), a short distance behind the clypeus (C, Clp) and just outside the anterior tentorial pit (at). The mandibles thus swing transversely on horizontal anteroposterior axes (G, a-c), as do the jaws of the amphipods and isopods among the Crustacea, and the jaws of the biting-and-chewing type in most of the pterygote insects. It is to be noted, however, that the doubly articulated mandible of the crustaceans and the pterygotes has its anterior articulation on the epistome, or clypeus.
The musculature of the lepismatid mandible includes the same muscles that operate the pendent mandible of the machilids, but the different articulation brings about a change in the action of the dorsal muscles on the jaw. In Ctenolepisma urbana (fig. 76 G) the anterior dorsal muscle of the machilid mandible (fig. 75 D, 1) is represented by two muscles (fig. 76 G, 1a, 1b) which are functionally dorsal abductors. The posterior dorsal muscle (2) becomes a dorsal adductor. Of the two ventral adductors, the proximal one (4) has its origin as in Machilidae on the anterior arms of the tentorium (C, 4), but the distal muscle (G, 3) of each mandible is attached separately on the base of the hypopharynx (D, 3), as it is in all pterygote insects in which this muscle is retained.
The tentorium of the Lepismatidae resembles that of Machilis insofar as the anterior and posterior parts are not united, but it approaches the orthopteroid type of tentorial structure in that the anterior arms are confluent in a large central plate (fig. 76 C, E). In Ctenolepisma (C) the plate is relatively short and only touches on the posterior bridge, but in Lepisma (E) the plate widely overlaps the supporting bridge beneath it. In Thermobia the median part of the bridge is a delicate band so closely adherent to the undersurface of the plate that the writer formerly (1951) described the two parts as united, though Chaudonneret (1950) had shown that they are separate. The invagination points of the anterior tentorial arms (at), as already noted, are in the ventrally inflected, anterior lower angles of the genae (C, Ge) just mesad of the articular condyles (c) of the mandibles; those of the posterior bridge (pt) are in the posterior part of the cranium between the attachments of the maxillae and the labium.
Fig. 76. Hexapoda—Thysanura.
A, Thermobia sp. B, Ctenolepisma urbana Slabaugh. C, same, section of head below level of tentorium. D, same, hypopharynx, cibarium, and pharynx, lateral. E, Lepisma sp., section of head below level of tentorium. F, Thermobia sp., hypopharynx, anterior. G, Ctenolepisma urbana Slabaugh, left mandible and muscles, dorsolateral. H, same, labium, ventral. I, same, base of left mesothoracic leg and adjoining parts, ventral. J, same, base of right mesothoracic coxa and pleuron, mesal. K, same, end of tarsus and pretarsus. L, same, eleventh abdominal segment (body segment 14), dorsal and ventral. M, same, end of male abdomen, ventral.
For explanation of lettering see pages 337–339.
The hypopharynx of the lepismatids is of particular interest because it has, fully developed, the typical structure of the hypo-pharynx in the lower pterygotes. The organ is a simple lobe projecting below the mouth (fig. 76 D, Hphy), supported by a U-shaped suspensorium (HS) on the proximal half of its anterior, or dorsal, surface, with oral arms (F, y) going through the mouth angles to give attachment to muscles from the frons (D, F, adhf), and a pair of lateral arms (F, x) on which the distal adductor muscles (F, D, 3) of the mandibles are attached. The preoral food cavity of the head runs back into a cibarial pocket (D, Cb) over the base of the hypopharynx, on the dorsal wall of which are attached a double row of dilator muscles (dlcb) arising on the clypeus (Clp) anterior to the frontal ganglion (FrGng) and its brain connectives. This entire structure is duplicated in the cockroach (fig. 79 F, G), but there is no suggestion of it in the Machilidae.
On the thorax each segmental pleural area of the lepismatids contains three fairly distinct superposed sclerites, or sclerotized folds, over the coxa (fig. 76 I, J). The uppermost sclerite is termed the anapleurite (apl), the middle one the catapleurite, or coxopleurite (cpl), and the one adjoining the coxa the trochantin (Tn). The nature and homologies of the pleural sclerites of Collembola and Thysanura have been the subject of considerable discussion (see Carpentier, 1946, 1947, and Barlet, 1950), but whether or not the pleural, or “subcoxal,” sclerites as developed in the apterygote insects and the chilopods are anything more than local sclerotization patterns is something the morphologists have not decided; it may be said, however, that there is no demonstrated reason for regarding them as remnants of a primitive subcoxal segment of the leg. The pretarsus of the lepismatid leg has a well-developed median dactyl (K, Dac), on which is attached the tendon (t) of the flexor muscle, and a pair of relatively long lateral claws (Un).
The abdomen of Lepismatidae has the same segmentation as that of Machilidae, but styli are present only on the eighth and ninth segments, or in some species on the seventh, eighth, and ninth. Eversible vesicles are absent. The ventral surfaces of the first eight segments in the male, and the first seven in the female, are covered by simple plates with no division into coxal and primary sternal plates as in the machilids. According to Heymons (1897), however, appendage rudiments in the form of lateral lobes of the sterna are present in the embryo of Lepisma on all the segments, but in the course of development they flatten out and finally unite completely with the sterna, except those of the eleventh segment, which elongate and become the cerci of the adult. On the ninth abdominal segment (twelfth body segment) of the adult male (fig. 76 M) is a pair of large, independent stylus-bearing plates (12Cx), evidently homologous with the coxal plates of the machilids (fig. 75 J, Cx), and between their bases is a simple, median penis (fig. 76 M, Pen). In the female similar coxal plates are present on both the eighth and the ninth segments and bear gonapophyses that form an ovipositor as in the machilids. The tenth abdominal segment (thirteenth body segment) is well developed in the lepismatids; its tergum (L, 13T) is produced into a broad lobe over the base of the caudal filament. Beneath it is concealed the small tergal plate of the last segment (14T), which bears the caudal filament. Behind the ventral region of this segment (14S) are the lateral anal lobes and a small median postanal lobe on the base of the caudal filament. The abdominal structure is thus seen to be essentially the same in both the Lepismatidae and the Machilidae.
PTERYGOTA: THE COCKROACH, PERIPLANETA
The cockroach is here presented as a representative of the great group of winged insects known as the Pterygota, though in various anatomical respects it is not a typical pterygote. Most cockroaches that have fully developed wings are known to fly, some better than others, but they do not have the usual flight mechanism, and it is a problem to understand how they fly at all. On the other hand, the locomotor function is highly developed in connection with the legs, and the pleural and sternal parts of the thorax have acquired an atypical structure apparently to accommodate freedom of leg action. The head and the feeding apparatus, however, are excellent subjects for a study of the generalized structure of the insect head and the fundamental mechanisms of the pterygote mouth parts. But again, since the female cockroach encloses her eggs in a capsule, the ovipositor does not have the typical form and mechanism of this organ in most egg-laying insects, and the external genitalia of the male cockroach represent a special type of development of the organs of copulation and insemination. Yet, the cockroach is regarded as a generalized insect, and modern species appear to differ but little from their ancestors of Carboniferous times, which are among the oldest of known insects. In studying the cockroach, therefore, we are dealing with an ancient type of insect, and the problems it presents in comparative insect anatomy make it all the more interesting and instructive. Moreover, the cockroach has become a favorite subject for the study of insect physiology and for experimental work in determining the effects and mode of action of insecticides. Periplaneta americana, described in the following pages, in particular thrives and multiplies well in the confinement of breeding cages and is now almost everywhere available for classroom study.
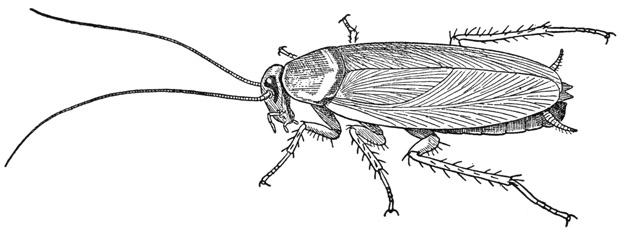
Fig. 77. Hexapoda—Pterygota. Periplaneta americana (L).
The cockroaches are often called simply “roaches,” but this abbreviation is not etymologically justified, because “cockroach” is a phonetic derivation from the Spanish cucaracha, and a roach is properly a kind of fish. In classical Latin the name blatta was applied to various insects, including cockroaches, but Linnaeus made it specifically a cockroach name, and from it the whole cockroach tribe is now called the Blattoidea by modem entomologists. Near relatives of the cockroaches are the mantids and the termites.
Periplaneta americana (fig. 77) is the largest of our common domestic cockroaches in the United States, but it is not so frequently found in houses as are some other species; it seems to prefer more roomy accommodations such as are offered by bakeries, mills, and restaurants, where there is also plenty of food and warmth. Our commonest home cockroach is the much smaller Blattella germanica, known as the waterbug, or Crotonbug, though the large black cockroach, Blatta orientalis, is a not infrequent visitor. In spite of their geographical names, the original homeland of these three cockroach species was probably Africa (see Rehn, 1945). In recent years another small cockroach with domestic habits, Supella supellectilium, the brown-banded cockroach, has been introduced and is already rather widely spread in many of the states. The giant cockroach, Blaberus craniifer, of Central America, occurs now in southern Florida. Besides the imported, so-called “domestic” cockroaches, there are also various native “wild” species that live out of doors, mostly in wooded places.
The Head
A cockroach at rest tucks its head back beneath the projecting margin of the shieldlike pronotum, so that from above little of the head is exposed. Museum specimens keep the head in this position, and consequently in pictures the cockroach nearly always has its head pulled back against the body with the face directed downward. Observation of a live cockroach in activity, inspecting a food source or in the act of feeding, however, will show that the head may be turned horizontally forward and that it is highly mobile in all directions. The head, in fact, is attached to the body by a large flexible neck and is pivoted on the ends of sclerites in the lateral neck walls.
In describing the cockroach head we may arbitrarily orient it in the hypognathous position, in which the facial aspect will be forward, and this direction we can then call “anterior” and the opposite “posterior.”
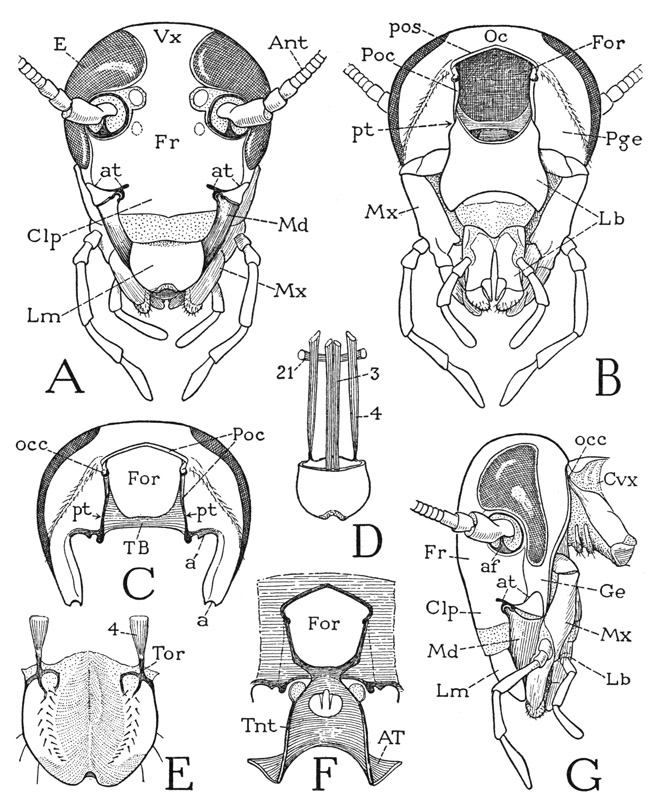
The head of Periplaneta is ovate in facial view (fig. 78 A) but is flattened anteroposteriorly (G). The antennae (A, Ant) arise from large, circular, dark-rimmed, widely separated membranous areas, or “sockets,” on the upper part of the face, which are surrounded laterally and dorsally by the compound eyes (E). The top of the head between the eyes is called the vertex (Vx), the region between and below the antennae is the frons (Fr), and a ventral extension from the frons between the bases of the mandibles represents the chypeus (Clp). In most insects the clypeus is set off from the frons by a transverse epistomal sulcus; in the cockroach, what may be taken to be the upper limit of the clypeus is marked by a pair of short lateral grooves (at) above the bases of the mandibles, which are the roots of the anterior arms of the tentorium. The distal part of the clypeal region is membranous and supports the labrum (Lm). At the sides of the clypeus are the mandibles (Md), which close behind the labrum, and behind the mandibles are seen the maxillae (Mx). In a nymphal cockroach the vertex of the head is divided by a median line that forks downward to the antennal sockets. This inverted Y-shaped line, present in most young insects, has commonly been called the “epicranial suture” and has been regarded as an important structural character of the head. Actually, however, it is a line of weakness in the nymphal cuticle where the latter will split at moulting to permit the ecdysis of the succeeding instar. At the last moult the cleavage line is not renewed in Periplaneta, though in some insects a trace of it may remain on the adult head.
The filamentous antennae of the cockroach consist each of three parts characteristic of the insect antenna in general. The large basal segment is the scape, beyond which is a much smaller segment known as the pedicel, followed by the long, multiarticulate flagellum. The scape is supported on a pivotal process, or antennifer (fig. 78 G, af), from the ventral rim of the antennal socket and is provided with three thick muscles arising on the tentorium, inserted on the base of the scape at three sides of the pivot, so that the antenna can be moved freely in all directions. The only muscles within the antenna are a dorsal and a ventral muscle arising in the scape, which are inserted on the pedicel and serve as a levator and depressor of the flagellum. The pedicel contains a chordotonal sense organ that probably registers the flagellar movements.
Fig. 78. Hexapoda—Pterygota. Periplaneta americana (L.). The head.
A, head, anterior. B, head, posterior. C, posterior wall of cranium, appendages removed, showing articulation of mandible at a, and of maxilla at a’. D, labrum and its muscles, anterior; 21, transverse muscle of frons. E, labrum, inner surface. F, posterior wall of cranium surrounding the neck foramen, and tentorium, anterior. G, head and neck, lateral.
For explanation of lettering see pages 337–339.
The large compound eyes of the cockroach have a lateral position on the head, but they are widened anteriorly above the bases of the antennae. On the upper part of the face, in the angles between the eyes and the rims of the antennal sockets, are two small, pale, oval areas in the position of the usual lateral ocelli of other adult insects, which are often called the “ocellar spots” of the cockroach. Beneath each cuticular disc is a small cellular body connected by a nerve with the protocerebral part of the brain. The structure of these organs in Blatta orientalis has been described by von Reitzenstein (1905) and by Haller (1907), but the accounts by the two writers are not entirely in agreement. In Periplaneta the cornealike cuticle is slightly convex outwardly but is of uniform thickness; in Blatta the central part of the disc is slightly thickened and thus has a lenslike form, as shown by von Reitzenstein, but in a dissection no such plug-shaped inner projection, such as that shown by Haller, is to be seen. Von Reitzenstein says that each organ is formed by invagination and has the structure of the “inverted” eyes of spiders; according to Haller, the structure of the adult organ does not suggest an origin by invagination. Both writers agree, however, that the body beneath the cuticular disc includes a central mass of cells produced individually into nerve fibers that come together to form the nerve trunk going to the brain, and that there is an entire absence of the usual elements of an ocular organ, such as rhabdoms and pigment. Apparently no experiments have been made on the function of the organs, but their position and brain connections leave little doubt that they represent lateral ocelli that have not developed in the usual manner. The mantis has three ocelli of ordinary structure.
Below the ocellar spots may be seen two other superficially similar but smaller spots. These spots, however, are merely the attachment points of a short transverse muscle on the inner surface of the frons (fig. 78 D, 21).
On the side of the head (78 G) a narrow area behind and below the eye, separated from the frons by a subocular groove, is the gena (Ge). The maxillae (Mx) and the labium (Lb) are seen to hang from the back of the head. Above their bases the head is attached to the body by the large neck (Cvx) and is supported on the ends of a pair of lateral neck plates.
On the back of the head (fig. 78 B) the most prominent feature is the large, rectangular foramen (For), generally called the occipital foramen, corresponding to the foramen magnum of the vertebrate skull, which connects the cavity of the head with that of the body. The rear part of the vertex over the foramen is known as the occiput (Oc), but in the cockroach the occipital region is not anatomically demarked. The areas of the cranium at the sides of the foramen are the postgenae (Pge). Very closely surrounding the foramen dorsally and laterally is a groove, the postoccipital sulcus (pos), which sets off a narrow marginal rim of the foramen termed the postocciput (Poc). Near the upper ends of the lateral parts of the postocciput are two small knobs, the occipital condyles (C, occ), by which the head is articulated on the lateral neck plates (G). Ventrally the neck foramen is closed only by the large basal plate of the labium (B, Lb), which is suspended by its lateral angles from the postocciput. On each side at the base of the labium is a slitlike depression (B, pt) in the lower end of the postoccipital sulcus. The two depressions are the posterior tentorial pits, marking the sites of the invaginations that formed the posterior part of the tentorium (C, TB).
When the labium and the maxillae are removed from the head (fig. 78 C), the posterior part of the tentorium is seen extending like a bridge (TB) through the back of the head between the two posterior tentorial pits. From the lower ends of the postocciput the cranial margins curve laterally and downward to the bases of the mandibles, bearing on each side near the foramen a small knob (a’) on which the maxilla is articulated, and ventrally a socket (a) for the posterior articular condyle of the mandible. It should be noted now that the maxillae hang from the cranial margins anterior to the postoccipital sulcus, and that the labium is suspended by its basal angles from the postocciput behind the sulcus. Between the two on each side is a posterior tentorial pit. The dorsal arc of the maxillary segment, therefore, would appear to be represented by the postgenae and the occiput, while the postocciput should be a remnant of the labial segment. If so, the posterior bridge of the tentorium is an intersegmental invagination.
The tentorium of the cockroach is formed as in most other insects by the union of a pair of anterior arms with the posterior bridge, and the bridge itself is formed by the union of two posterior arms. The invagination points of the anterior arms vary in different insects, but the posterior arms are always ingrowths from the postoccipital sulcus. In the cockroach, as in Lepismatidae (fig. 76 E), the anterior tentorial arms are confluent in a large central plate (fig. 78 F, Tnt), but the union is not complete since there is left an oval aperture for the passage of the nerve connectives from the brain to the ventral ganglion of the head. Since the insect head has no sternal sclerotiza-tion, the tentorium serves as a substitute for bracing the lower edges of the cranial walls, and for the attachment of the ventral muscles of the mouth parts. The tentorial plate of the cockroach slopes steeply upward and backward between its anterior and posterior supports. The pharynx lies above it and turns down to the mouth between the anterior arms.
The neck of the cockroach is a complex structure enabling the insect to move the head freely on the body. On each side are two large neck sclerites flexibly joined to each other, which may be termed the dorsal and ventral lateral cervical plates (fig. 81 D, levpls), though the two of the lower pair almost come together on the ventral side of the neck. On the dorsal surface are two small, weakly sclerotized dorsal plates, one behind the other, the first of which is attached to the postoccipital margin of the head. On the ventral surface are two narrow gular sclerites (gu), which are mere transverse ridges. The upper ends of the dorsal lateral plates are articulated on the occipital condyles of the head (fig. 78 G, occ) and are the fulcral supports on which the head moves. The neck musculature, as described by Carbonell (1947), includes muscles that retract the head against the thorax, muscles that protract the head by extending the neck, and muscles that tilt the head up or down on the neck fulcra, or turn it from side to side. In the retracted position of the head, the lateral neck plates take an oblique position against the prothorax, and the two of each side are strongly elbowed on each other. Extension of the neck is produced evidently by muscles from the head and from the pronotum attached on the lateral neck plates, the pull of which flattens the angle between the plates of each pair and thus protrudes the head. Few animals except insects can actually stretch their necks. Muscles from the thorax attached dorsally on the postoccipital ridge of the cranium, and others attached ventrally on the tentorium, serve antagonistically to tilt the head up and down on the cervical fulcra and probably produce also lateral movements. Carbonell lists ten pairs of neck muscles in Periplaneta concerned with movement of the head.
The nature of the insect neck is somewhat of a morphological problem. The anatomical relations of the posterior parts of the cranium seem to indicate that the postoccipital ridge and the posterior arms of the tentorium are inflections between the maxillary and the labial segments. If so, the narrow postocciput is a sclerotic remnant of the labial segment, and the primitive intersegmental line between the labial and prothoracic segments should be somewhere in the neck. The head muscles, however, go from the prothorax to the postoccipital ridge and the tentorium, and thus would seem to be continuous through two primary segments. There is no evidence whatever that the neck itself represents a segment, and the segmental status of the cervical sclerites is not clear.
The Feeding Apparatus
The parts of the insect concerned with the acquisition and ingestion of food include the three pairs of segmental appendages of the head associated with the mouth, and also the labrum, the inner, or so-called “epipharyngeal,” surface of the clypeus, and the hypopharynx. The appendicular organs are the mandibles, the maxillae, and the labium.
The Labrum— The labrum (fig. 78 A, Lm) is a flat, hollow lobe of the head suspended from the clypeus. It is movable by muscles inserted on its base, and in the cockroach it is freely retractile because of the wide membranous connection with the clypeus. The la-bral muscles (D) arise on the upper part of the frons, one pair (3) being median and inserted anteriorly on the base of the labrum, the other pair (4) inserted laterally and posteriorly. The posterior muscles are attached on special sclerites, known as the tormae (E, Tor), of the inner wall of the labrum. The varied movements of the labrum by a live cockroach suggest that the muscles may act as antagonists in different combinations. In most insects the labrum is compressible by a pair of interior muscles between its outer and inner walls; in the cockroach these muscles take a transverse position.
The Mandibles— The mandibles of Periplaneta are strongly toothed jaws (fig. 79 A) suspended from the lateral margins of the cranium (B) so that they close upon each other within the preoral food cavity of the head between the labrum in front and the hypopharynx behind. On the mesal surface at the base of each mandible is a small, flat molar area, and proximal to it is a thin membranous flap. When the jaws are closed with the molar areas in contact, the teeth of the left mandible overlap anteriorly those of the right, and the two basal flaps project over the food trough, or sitophore (F, Sit), on the base of the hypopharynx.
Each mandible is attached to the head by an articular membrane all around its base, but it is specifically hinged to the cranial margin by an anterior and a posterior point of articulation on the outer side of its base (fig. 79 B, c, a). The mandibular articulations are on the free surface of the mandible and are of the ball-and-socket type of structure, but the parts are reversed in the two, the condyle of the anterior articulation (c) being on the cranium, that of the posterior articulation (a) on the mandible. The posterior articulation represents the single primary articulation of a mandible such as that of the Machilidae (fig. 75 B, a).
The musculature of the cockroach mandibles includes four distinct muscles for each jaw (fig. 79 C), two arising dorsally on the cranium, one on the tentorium, and one on the hypopharynx. Of the dorsal muscles, one is a relatively small abductor (27) arising on the side of the head and inserted by a slender tendon in the membrane at the base of the outer side of the mandible; the other (28) is a huge adductor of several bundles of fibers converging upon a broad tendon (t) attached at the inner angle of the base of the mandible. The power of the adductor muscle is increased by its long leverage mesad of the mandibular hinge on the cranium. The hypo-pharyngeal muscle of the mandible (29) has a narrow origin on a small branch (x) of the hypopharyngeal suspensorium, and its fibers spread to the inner surface of the lateral wall of the jaw. The fourth muscle is a small bundle of fibers (30) from the underside of the anterior arm of the tentorium (AT) to the posterior wall of the mandible. The function of the hypopharyngeal and tentorial muscles of the insect mandibles is not clear; these muscles, however, represent the important ventral adductors of a primitive mandible, which, though much reduced, are still retained in most of the lower insects, but the functions of adduction and abduction have been largely taken over by the dorsal muscles, which are the rotators of the primitive, singly articulated jaw. In the higher insects with biting and chewing mandibles, the ventral muscles have been eliminated, and the jaws are operated entirely by the dorsal muscles. The same evolution of the mandibular mechanism has taken place in the amphipods and isopods among the Crustacea. The mandibles of the cockroach can only bite and chew, they cannot reach out and grasp the food; it is the function of the maxillae to bring food back to the mandibles.
Fig. 79. Hexapoda—Pterygota. Periplaneta americana (L). The mandibles and the hypopharynx.
A, mandibles, anterior. B, left mandible and lower part of head, lateral. C, diagrammatic cross section of head, anterior, showing tentorium, mandibles and their muscles, and hypopharynx. D, lingual lobe of hypopharynx, posterior, showing opening of salivary duct on its base. E, basal part of hypopharynx supported on labium, showing hypopharyngeal muscles and salivary duct. F, hypopharynx and pharyngeal part of stomodaeum, with frontal ganglion on lower end of pharynx. G, diagrammatic longitudinal section of lower part of head, showing position of hypopharynx in preoral cavity.
For explanation of lettering see pages 337–339.
The Maxillae— The insect maxilla retains in its structure something of its leg origin. The large base of the appendage (fig. 80 A), representing the coxa, supports a jointed palpus, which in the cockroach has five segments comparable to those of an arthropod leg with two trochanters, a knee bend between the femur and the tibia, and a tarsus, but lacking the apical segment, or pretarsus. The basal part of the maxilla bears mesad of the palpus two large endites, an inner lacinia (Lc) and a lateral galea (Ga), and is itself divided by an elbowlike joint into a proximal cardo (Cd) and a distal stipes (St). The cardo and stipes clearly do not represent segments of the maxilla; there are no muscles between them, and the two parts have a common, wide, mesal opening from the head. The single articular support of the maxilla on the head is by means of a small process on the base of the cardo (D, a’) that articulates with the cranial margin on the back of the head (fig. 78 C, a’); otherwise both cardo and stipes have only a wide membranous connection with the head (fig. 80 A), which allows the maxilla a free movement on the cardinal articulation. The elbow joint between the cardo and the stipes is merely a mechanical device that permits the maxilla as a whole to be protracted and retracted.
The lacinia (fig. 80 A, Lc) is a rigid, flattened lobe tapering distally and ending with two sharp, incurved teeth, proximal to which is a weak, subapical process. The inner margin bears a fringe of long hairs, from which the lacinia gets its name. The galea (Ga) is a relatively soft, thick lobe with a hoodlike apical pad that partially encloses the end of the lacinia. The galea is so named from its fancied resemblance to a helmet. The galea and the lacinia are each individually movable. The galea has a large muscle (B, 42) from the base of the stipes; a muscle (41) attached on the base of the lacinia has its origin on the side of the stipes. The hinge of the lacinia on the stipes (A, h), however, allows the lacinia only an anteroposterior movement on the stipes, while keeping it firmly in position against the galea. The maxillary palpus is movable as a whole by two basal muscles (C, 35, 36) arising in the stipes; the segments are musculated as shown in the figure.
The muscles of the maxillary base correspond with the muscles of the mandible insofar as they include dorsal muscles arising on the cranial wall and ventral muscles arising on the tentorium. In Periplaneta (fig. 80 B) there is a small dorsal muscle (31) inserted on the inner end of the cardo just beyond the cranial articulation (a’) and a much larger and longer dorsal muscle (32) inserted at the base of the lacinia. The ventral muscles of the maxilla, arising on the undersurface of the tentorium, include several compact bundles of fibers (33) going to the outer end of the cardo, and a large group of muscles (34a, b, c) attached on the posterior margin of the stipes. The ventral muscles of the maxilla represent the adductors of a generalized appendage, but the distal parts of the insect maxillae lie close against the sides of the hypopharynx (F, Hphy). Consequently the mesal pull of the tentorial muscles (E, 33, 34) flattens the angles between the cardines and stipites, with the result that the maxillae, instead of being adducted, are protracted beyond the end of the hypopharynx. Retraction then is effected by the contraction of the long cranial muscles (B, 32) of the stipites.
The maxillary movements are easily observed in a live cockroach while feeding. They are exactly comparable to the movements of a pair of human arms flexed outward at the elbows, with the hands held together, and successively protruded and retracted. The movements are always the same regardless of the stimulus; whatever is placed between the maxillary lobes elicits the same response. During feeding, particles of food are grasped by the lacinial teeth, enclosed by the galeal lobes, and brought back to the mandibles for mastication. In addition to their function in feeding, the maxillae are used for cleaning the antennae, the palpi, and the front legs.
The Labium— The insect labium, or at least a part of it, represents the second maxillae of other arthropods. In its generalized form, as we have seen it in the Thysanura (fig. 76 H), the labium consists fundamentally of two parts, an immovable basal part implanted on the back of the head, and a distal part, which bears the palpi and is movably suspended on the base. In the cockroach the labial structure (fig. 80 G) is the same as in the thysanurans, except that the free distal part of the organ has a wide membranous connection with the base. The labium as a whole is thus divided into a proximal part conventionally termed the postmentum (Pmt) and a distal part called the prementum (Prmt). In the cockroach and various other insects, the postmental region contains two plates distinguished as the mentum (Mt) and the submentum (Smt). The submentum of the cockroach is a large plate of the posterior head wall just below the neck foramen (fig. 78 B); the mentum is a small, weakly sclerotized plate (fig. 80 G, Mt) in the membranous distal part of the postmental region. In some insects the mentum occupies the entire space between the prementum and the submentum, while in others there is only a single postmental plate. The prementum of the cockroach (Prmt) much resembles a pair of maxillae united at their bases, except for the lack of cardines. Each half of the body of the prementum (St) evidently represents the stipes of a maxilla. The prementum bears a pair of three-segmented palpi (Plp) and four apical lobes, the two median lobes being the glossae (Gl), the outer lobes the paraglossae (Pgl). The four lobes together are sometimes termed the ligula.
The labial musculature (fig. 80 H) includes muscles that move the prementum as a whole and individual muscles of the palpi, the glossae, and the paraglossae, which take their origins in the stipital lobes of the prementum. The premental muscles include two pairs of long slender lateral muscles (43, 44) from the posterior part of the tentorium, inserted distally and proximately on the prementum, and a pair of median muscles (45) from the submentum to the base of the prementum. The median muscles serve as retractors of the prementum; they always cross over the mentum, showing that this plate belongs to the postmental region of the labium. The mentum and the submentum have no muscles of their own, and they seem to have no counterparts in the maxillae. Some writers have regarded the submentum as the sternal plate of the labial segment of the head, but embryologists say it is derived from the embryonic labial appendages. Again, it has been supposed that the postmental sclerotization represents the cardines of the maxillae, but the cardines are movable parts of the maxillae and have their own muscles from both the cranium and the tentorium.
The functional underlip of the insect is the so-called prementum. It serves passively to close the preoral food cavity of the head behind the maxillae and the hypopharynx but takes no particularly active part in feeding, except that the glossae and paraglossae prevent the loss of food particles from the mandibles. The strongly musculated palpi (fig. 80 I), however, are extremely active, though their function appears to be mainly sensory.
Fig. 80. Hexapoda—Pterygota. Periplaneta americana (L.). The maxillae and the labium.
A, right maxilla, posterior. B, right maxilla and muscles, anterior. C, maxillary palpus and muscles. D, maxillary cardo and muscle. E, diagram showing relation of maxillae to hypopharynx and protractor action of adductor muscles of maxillae, anterior. F, maxillae in position of retraction against sides of hypopharynx, anterior. G, labium, posterior. H, labium and its muscles, anterior. I, labial palpus and its muscles.
For explanation of lettering see pages 337–339.
To anyone who holds that the application of scientific terms should be consistent with their meanings, it is obvious that our current nomenclature for the parts of the insect labium is highly inconsistent. Since mentum means “chin,” and labium means “lip,” the term labium should be restricted to the true underlip of the insect, which is the part called prementum (literally, “prechin”). The postmentum then might not inappropriately be termed the mentum, or mentum and submentum if the insect has a “double chin,” but anatomically it is quite incongruous that the “chin” should be a part of the “lip.”
The Preoral Food Cavity and the Hypopharynx— The functional mouth cavity of the insect, into which the food is first received and where it is masticated by the jaws, is merely the space enclosed between the clypeus and labrum in front, the prementum of the labium behind, and the mandibles and maxillae on the sides. In an anatomical sense this space (fig. 79 G, PrC) is not a “mouth cavity,” or “buccal cavity,” as it is often called; it is properly a preoral food cavity, since the true mouth (Mth) is at its inner end and opens directly into the stomodaeal pharynx (Phy). The inner wall of the food cavity in the cockroach slopes downward and posteriorly from the mouth to the base of the prementum, and from it projects the large, tonguelike hypopharynx (Hphy). The mandibles close upon each other in the food cavity between the labrum and the hypopharynx. The part of the cavity proximal to the mandibles, therefore, serves as a food receptacle, or cibarium (Cb), for the masticated food passed back from the jaws. Between the base of the hypopharynx and the base of the prementum is a pocket, the salivarium (Slv), into which opens the salivary duct (SlDct).
The hypopharynx of the cockroach (fig. 79 F, Hphy) has a long sloping base on the oblique inner wall of the preoral cavity (G), and only its distal part is a free lingual lobe (F, Lin). In the lateral walls of the lobe is a pair of elongate sclerites, the tapering inner ends of which curve posteriorly and unite with each other behind the orifice of the salivary duct (D, SlO) in an arc that rests on the base of the prementum (G, hf) and serves as a fulcrum for the movements of the hypopharynx. Though it is commonly said that the opening of the salivary glands lies between the base of the hypopharynx and the base of the prementum of the labium, in the cockroach and some other lower insects the salivary duct opens actually on the base of the hypopharynx. This fact was noted in the cockroach by Miall and Denny (1886, p. 45), who says: “The common duct of the salivary glands enters the lingua, and opens on its hinder surface.” As is well known, in such insects as Hemiptera, Diptera, and Siphonaptera the duct traverses the hypopharynx to open at its tip.
The anterior wall of the basal part of the hypopharynx, between the free lingual lobe and the mouth (fig. 79 F), is margined by a pair of rodlike sclerites (HS) that curve mesally at their lower ends in the base of the lingua, while their upper parts are continued as slender oral arms (y) that run through the angles of the mouth (F, G) to give insertion each to a pair of muscles, one muscle (13) arising dorsally on the frons, the other (14) ventrolaterally. The hypopharynx is thus hung from the cranium by a long U-shaped suspensorium. Small lateral branches (x) of the suspensorial arms give attachment to the hypopharyngeal muscles of the mandibles (C, F, 29). Between the suspensory arms the surface of the hypopharynx is depressed, forming a troughlike food channel, or sitophore (F, Sit), widening upward into the mouth.
The principal movements of the hypopharynx are production and reduction of the lingual lobe on the labial fulcrum (fig. 79 G, hf). The productor muscles include the dorsal frontal muscles of the suspensorial arms (F, G, 13) and a pair of long ventral muscles (E, 16) from the tentorium attached to the proximal parts of the lateral lingual sclerites, and perhaps also the pair of muscles (17) from the lateral branches (x) of the suspensorial arms to the posterior wall of the lingua. Antagonistic to the productors are the ventral frontal muscles of the suspensorium (F, G, 14) and a muscle from the labium (E, 19) attached on the lingual sclerites opposite the tentorial muscles (16). If the base of the hypopharynx is brought against the inner wall of the clypeus, the cibarium (G, Cb) will become a closed pocket, which can be expanded by the compressor muscles of the clypeus (5a, 5b) and contracted by the transverse muscles on the inner clypeal wall (F, G). There is little doubt that the cibarium thus acts as a sucking pump when the cockroach drinks liquids. It would seem that by some similar activity of the cibarium food stored on the sitophore of the hypopharyngeal base must be forced back into the mouth, but the exact mechanism of food ingestion is not clear and has not been observed. The hypopharynx is not retractile toward the mouth, except to the extent that the base of the lingua moves upward with its forward movement on the labial fulcrum. The membranous lobes on the bases of the mandibles (A) very probably serve to press the masticated food back into the sitophore of the hypopharynx, but the food is still a long way from the mouth. In most of the insects that feed entirely on liquid food, the preoral cibarium has been developed into an efficient sucking pump operated by the clypeal muscles.
The Thorax
When the ancestors of the flying insects developed wings, the thorax was already differentiated as the locomotor center of the body, but it now had new responsibilities thrust upon it, and to meet them it had to undergo a considerable amount of reconstruction. Hence we find that the thoracic segments of flying insects, particularly the wing-bearing segments, have many structural features that make them quite different from the ordinary leg-bearing segments of other arthropods and from the simplified legless segments of the insect abdomen. Insect wings are merely flat outgrowths of the integument from the lateral parts of the dorsum of the mesothorax and the metathorax, and as such they may be compared to the tergal folds of the carapace that cover the gills in decapod crustaceans (fig. 41 D, tf); if the crustacean branchiostegites were extended outward from the body they would have the position of insect wings. In either case, the part of the body wall above the fold becomes specifically the tergum, and the part between the fold and the base of the leg is called the pleuron. In the decapod thorax the simple pleuron is the inner wall of the gill chamber, on which the legs are articulated; in the insect the pleura of the mesothorax and the metathorax, in addition to supporting the legs, furnish also the supports for the wings and give attachment to important wing muscles. The general similarity of the propleuron to the pleura of the winged segments might suggest that the fundamental structure of the thoracic pleura was established before wings were developed. On the other hand, it may be that in the glider stage of the wing evolution there were wing folds (paranotal lobes) also on the prothorax, since some ancient fossil insects do have such folds on the prothorax in addition to fully developed wings. However, there is no end to the possibilities of speculation as to how insects acquired their wings, but there is no definite information on the subject, inasmuch as the very oldest of known winged insects already possessed two pairs of perfect wings. The tergum of a winged segment has undergone more extensive adaptational modifications than have the pleura, because the wings are movably supported on its lateral margins and the tergum itself becomes an important part of the mechanism of wing movement.
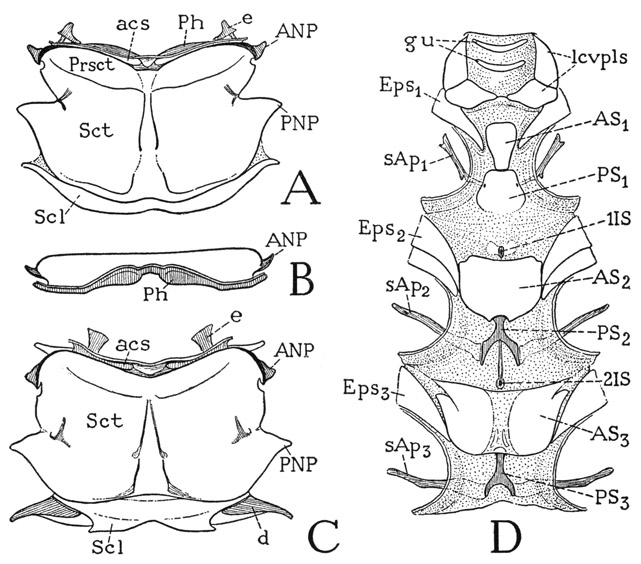
Fig. 81. Hexapoda—Pterygota. Periplaneta americana (L.). Tergal and sternal plates of the thorax.
A, mesonotum, dorsal. B, metanotum, anterior. C, metanotum, dorsal. D, ventral surface of neck and thorax.
For explanation of lettering see pages 337–339.
The thorax of the cockroach is in some respects more generalized than that of most other flying insects because the wing-bearing terga play a smaller part in the mechanism of flight; but, on the other hand, the pleura of the three thoracic segments have specialized features adaptive to a free movement of the legs. The thoracic musculature of the cockroach is strongly developed in relation to the legs, but some of the usual flight muscles of other insects are much reduced or absent. A fully illustrated account of the thoracic musculature of Periplaneta is given by Carbonell (1947).
The Thoracic Terga— Though the word tergum, of Latin origin, is the general term given to the back plate of an arthropod body segment, the thoracic terga of insects are commonly called nota, from the Greek noton, because notum more properly combines with the Greek prefixes pro, meso, and meta that designate the three thoracic segments.
The pronotum of Periplaneta is a large, slightly convex, triangular plate with rounded corners set like a shield on the back of the prothorax. Its wide free margins overlap the retracted head anteriorly, the bases of the wings posteriorly, and the prothoracic pleura on the sides. The central disc of the shield gives attachment to muscles of the head, the neck, and the prothoracic pleura, coxae, and trochanters.
The mesonotum and the metanotum of Periplaneta are similar to each other in size and shape. Each plate is a wide, almost flat, rectangular sclerotization of the dorsum of its segment, with irregular lateral margins (fig. 81 A, C), and is divided into a large anterior plate and a smaller posterior plate. The major part of the anterior plate is termed the scutum (Sct), but a short anterior part set off by a pair of weak transverse grooves is designated the prescutum (Prsct). The scutum is strengthened internally by a narrow, median V-shaped ridge indicated by two anteriorly convergent grooves on the outer surface. The narrow posterior plate behind the scutum of each tergum is the scutellum (Scl). The front margin of the prescutum is deflected into a deep, transverse groove (acs), termed the antecostal sulcus because in general it forms an internal submarginal ridge, or antecosta, of the tergum, on which the longitudinal intersegmental dorsal muscles are attached. In the wing-bearing segments of insects with large dorsal muscles the antecostae are produced into deep, usually bilobed plates, termed phragmata. The dorsal muscles of the cockroach, however, are very small, and each phragma is a relatively low, bilobed infolding from the antecostal sulcus (A, B, Ph). The antecostal sulci mark the true intersegmental lines of the thorax, so that the narrow anterior lip of each groove really belongs to the preceding segment, as does also the functional “intersegmental membrane” before it. In most strong-flying insects the precostal lip of both the metanotum and the first abdominal tergum is enlarged to form a wide plate, the postscutellum, or postnotum, which takes the place of the conjunctival membrane and firmly connects the adjoining terga. In the cockroach each intertergal membrane contains a pair of small connective sclerites (A, C, e) between the consecutive terga.
The structure of the lateral margins of the mesonotum and the metanotum of Periplaneta is characteristic of that of winged insects in general. Anteriorly on each side is a small but strongly developed anterior notal wing process (fig. 81 A, C, ANP), behind which is a small incision of the tergal margin, followed by a deeper indentation, and then a large triangular projection (PNP), which is the posterior notal wing process. The relation of these parts to the wing will be shown in the section on the wings. The scutellum of each segment (Scl) is produced laterally into tapering arms with which are connected the posterior margins of the wings. In the metathorax the scutellar arms (C, d) are detached sclerites supporting the basal membranes of the wings (fig. 84 D).
The Thoracic Pleura— The pleural areas of the thoracic segments of the cockroach do not closely conform in structure with the typical pleuron of a wing-bearing segment, shown diagrammatieally at A of figure 82, but there is little difficulty in identifying their parts by comparison with the diagram. The sclerotic pleural wall is always marked by a deep, vertical or oblique groove, the pleural sulcus (A, PlS), that forms a strong ridge on the inner surface between the coxal articular process (a) below and the wing-supporting process (WP) above. The part of the pleuron in front of the groove and its ridge is the episternum (Eps), the part behind it, the epimeron (Epm). The episternum may be extended ventrally before the coxa to the sternum (S). Between the coxa and the precoxal arm of the episternum is a separate sclerite, the trochantin (Tn), closely connected with the episternum dorsally and articulated with the anterior margin of the coxa ventrally (c). Resting on the upper end of the episternum before the wing process is a small sclerite (Ba) termed the basalare, and in the pleuroalar membrane behind the wing process is a sclerite distinguished as the subalare (Sa). The pleural structure in the prothorax is simpler than that of the mesothorax and metathorax because of the absence of the wing process and the epipleural sclerites.
In the prothorax of Periplaneta (fig. 82 B) the pleural sulcus (PlS) runs obliquely upward and forward from the coxal articulation (a) to the tergum. Behind the sulcus is an irregular epimeral sclerotization (Epm); in front of it is the episternum and the trochantin. The episternal sclerotization (Eps), however, is broken up into several parts, and the trochantin (Tn) appears to be divided into a large, triangular dorsal plate partially united with the episternum and a narrow ventral sclerite articulating below with the coxa. At least, the prothoracic trochantin is thus interpreted in the cockroach by Cramp-ton (1927) and in the mantis by Levereault (1936). On the other hand, Fuller (1924) attributes the much smaller dorsal sclerite of the winged termite to the episternum. The cockroach itself is noncommittal, but the trochantinal muscles of Periplaneta are shown by Carbonell (1947) to be all inserted on the lower sclerite.
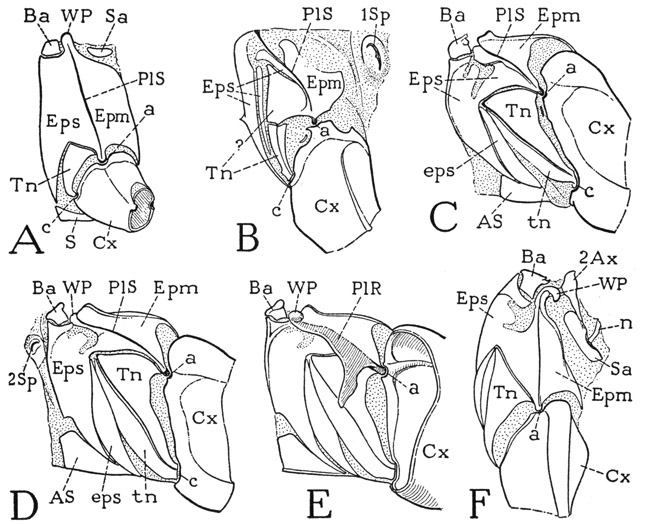
Fig. 82. Hexapoda—Pterygota. Periplaneta americana (L.). The thoracic pleura.
A, diagram of principal parts of a generalized pleuron of a winged insect. B, propleuron of Periplaneta with base of coxa and first spiracle, left side. C, mesopleuron and base of coxa, left side. D, metapleuron with base of coxa and second spiracle, left side. E, right metapleuron, inner surface. F, left metapleuron and base of coxa, dorsal, with subalar sclerite and second axillary of wing base.
For explanation of lettering see pages 337–339.
In the mesothorax (fig. 82 C) the pattern of the sclerotization is somewhat different from that in the prothorax, but the episternum (Eps) and the epimeron (Epm) are to be identified as the parts lying respectively before and behind the oblique pleural sulcus (PlS), The episternal surface is more continuously sclerotized than is that of the prothorax and is prolonged ventrally in a broader extension to the sternum. The trochantin (Tn) is a long, triangular sclerite; its anterior part is set off as a narrow marginal band (tn) that may be termed the trochantinal apotome. A similar episternal apotome (eps) is cut off from the posterior margin of the ventral extension of the episternum and partly overlaps the trochantinal apotome. The wing process of the mesopleuron is not fully visible in the lateral view (C), but in front of it is a distinct basalar sclerite (Ba) flexibly attached on the upper end of the episternum. A subalar sclerite lies in the pleuroalar membrane above the epimeron but is not shown in the figure.
The pleuron of the metathorax (fig. 82 D) closely resembles that of the mesothorax and will need no special description. It may be noted, however, that the pleural sulcus becomes increasingly oblique in the successive thoracic segments. A dorsal view of the metapleuron (F) gives a better view of the wing process (WP) than the side view and shows something of the relation of the pleuron to the wing base. Closely connected with the wing process is the second axillary sclerite of the wing (2Ax), with its posterior arm attached to the elongate subalar sclerite (Sa). Also attached on the subalare is a ventral stalk (n) of the third axillary, but these features will be more fully described in connection with the wing mechanism. On the inner surface of the pleuron (E) is seen the strong pleural ridge (PlR) along the line of the pleural sulcus on the outer surface (D, PlS), forming at its lower end a condyle for the coxal articulation (a) and ending above in the wing process (WP), Near the coxa the ridge is produced into a large apodemal arm. A similar pleural ridge and arm are present also in the prothorax and the mesothorax.
The Thoracic Spiracles— The cockroach, in common with most other insects, has ten pairs of spiracles, two pairs of which are on the thorax and eight on the abdomen. The two sets of spiracles, however, are quite different in structure, particularly in the nature of the closing mechanism. The spiracles of the first thoracic pair (fig. 82 B, 1Sp) lie between the pleural areas of the prothorax and the mesothorax just in front of the bases of the forewings; those of the second pair (D, 2Sp) are similarly situated, though at a somewhat lower level, between the mesothorax and the metathorax. The spiracles of each pair on the thorax are presumed to belong to the segment behind them, since a truly intersegmental position is an unlikely place for a respiratory orifice.
The first spiracles of Periplaneta are much larger than any of the others. Each of these spiracles appears externally (fig. 85 G) as an elongate oval mound with an oblique median slit; the wider anterior lip is entirely soft; the narrower posterior lip has a beveled edge with a strongly sclerotized margin. The outer slit opens into an atrial chamber from which the tracheae are given off. Below the middle of the spiracle the thickened edge of the posterior lip is produced in an obliquely transverse arm through the floor of the atrium to the base of the inner wall of the anterior lip, where it gives insertion to a fan-shaped muscle (mcl) arising anteriorly at the base of the spiracle. This muscle serves to close the spiracle by drawing the beveled edge of the posterior lip against the soft edge of the anterior lip. Four large tracheae are given off directly from the atrium above the muscle arm, and below it arises a single trachea that immediately divides into two branches.
The second spiracle (fig. 85 H) is smaller than the first and appears externally as a somewhat circular mound with an obliquely longitudinal crescentic slit. The larger and relatively rigid dorsal lip is hood-shaped with a strong, concave margin; the posterior lip is a soft, thick flap with a rounded margin. At the lower, anterior angle of the spiracular cleft is attached the narrow end of a fan-shaped muscle (mcl) arising ventrally at the base of the spiracle. The pull of this muscle closes the spiracle by bringing the ventral lip against the dorsal lip, as may be demonstrated by pressing down with a needle on the point of the muscle insertion. The spiracular orifice leads into a cup-shaped atrium, from which arise several tracheal trunks.
The Thoracic Sterna— The ventral surface of the thorax in the cockroach is largely membranous (fig. 81 D), but in each segment there are to be distinguished two sternal plates, one anterior, the other posterior. The plates have been called respectively the sternum, or eusternum, and the sternellum, or, in the more commonly used terms of Crampton (1927), the anterior plate is the basistemum, the posterior plate the furcasternum. The name “furcastemum” is based on the fact that in most of the higher insects the second plate carries a two-pronged apodemal fork. In the cockroach and in various other of the more generalized insects, however, the sternal apodemes are widely separated arms (D, sAp) that in no sense constitute a “furca.” To avoid ambiguity in describing the cockroach, therefore, we may call the anterior sternal plate of each segment the antesternite (AS) and the posterior plate the poststernite (PS). In the prothorax the antesternite (AS1) is a relatively small, pear-shaped plate, the somewhat larger poststernite (PS1) carries the sternal apodemes (sAp1) on its lateral margins. In the mesothorax and the metathorax the antesternites (AS2, AS3) are large, shield-shaped plates, and the poststernites (PS2, PS3) are small Y-shaped sclerites, each attached by the stem to the preceding antesternite and bearing the long sternal apodemes on the ends of its divergent arms. Between the prothorax and the mesothorax, and between the mesothorax and the metathorax, are two very small intersegmental sternites (1IS, 2IS), commonly called the spinisternites because each supports a small spinelike median apodeme.
The Legs
The legs of the cockroach are typical of the legs of insects in general. Each leg (fig. 83 A) has six segments. There is only one segment in the trochanteral region; a sharp knee bend takes place at the femorotibial joint; the tarsus is subdivided into five tarsomeres; the pretarsus bears a pair of lateral claws and a median adhesive lobe. Only in the dragonflies among the insects are there two trochanteral segments; there are never more than five tarsal subsegments, but the number may be fewer; the pretarsus undergoes various modifications, but a median claw is present in adult insects only in the apterygotes.
The legs of Periplaneta are essentially all alike in structure. When the insect is at rest (fig. 77), the coxae lie back against the sides of the body, with the first legs directed forward, the hind legs stretched out posteriorly, and the middle legs taking whatever position is convenient. During activity the slenderer and more mobile fore coxa are turned downward and the first legs are directed forward. These legs appear to determine the course of the insect when walking or running, while the others serve as the chief organs of locomotion. The front legs hold up the fore part of the body and the head during feeding, and when an antenna needs cleaning, one of them reaches up and pulls the base of the antenna down to the lobes of the maxillae. The coxae of the middle and the hind legs show little activity during ordinary walking, the principal movements of these legs being at the coxotrochanteral and the femorotibial joints. Yet the coxae are strongly musculated and they can turn forward and backward on their pleurotrochantinal hinges (fig. 82 C, D, a, c). Furthermore, as has been well explained by Carbonell (1947), each coxa can be adducted on its pleural articulation (a) by the trochantin, which swings mesally and forward on its dorsal connection with the episternum, while the trochantinal apotome folds beneath the episternal apotome. The trochantin thus appears to be an important part of the mechanism for the coxal movement. Carbonell has shown that there are six muscles from the tergum and the episternum attached on the trochantinal apotome. The greater mobility of the fore coxa is due to the freely flexible connection of the coxa with the pleuron by the small lower trochantinal sclerite (fig. 82 B), on which all the muscles of the trochantin are attached.
For a study of the structural details and the mechanism of the cockroach legs we may take one of the hind legs because of its greater size. In the usual position of the hind leg, as it is stretched out posteriorly in a horizontal plane (fig. 77), the true anterior surface is ventral and the posterior surface dorsal, but for descriptive purposes the leg will be assumed to be extended laterally from the body, so that “anterior” refers to the surface ordinarily turned downward and “posterior” to the opposite surface.
The large flat coxa of the hind leg of Periplaneta (fig. 83 A, Cx), is hinged almost transversely on the body between its pleural and trochantinal articulation (a, c). On each coxa are attached ten muscles from the body, most of which are either promotors or remotors, but the coxal muscles include a large muscle from the upper angle of the coxa (the meron) to the subalar sclerite of the wing base, which evidently is an important wing muscle. The movements of the coxa regulate the position of the leg on the body, but the principal movement of the leg as a whole is at the coxotrochanteral joint.
The trochanter, though a very small segment closely attached to the base of the femur (fig. 83 A, Tr), is the most strongly musculated segment of the leg. It rocks on an anteroposterior hinge on the end of the coxa between strong anterior and posterior points of articulation (B, o), and its movements cause a flexion or extension of the leg in the plane of the coxa. As shown by Carbonell (1947), there are three trochanteral depressor muscles and three levators. The principal depressor consists of five branches, three of which are body muscles arising on the tergum, the basalar sclerite of the pleuron, and the apodeme of the sternum, all of which are inserted on a large, flat, spatulate apodemal tendon (B, 177) attached on the proximal end of the trochanter. The two other depressor muscles arise anteriorly and posteriorly in the coxa and are inserted on separate anterior and posterior tendons (178, 179). The three antagonistic levator muscles have their origins in the coxa and are inserted on three slender tendons (180, 181, 182) attached to the trochanter distal to the axis of rotation. The depression of the leg at the coxotrochanteral joint is the force that lifts the body when the tarsus is pressed against a support, or enables the leg to give a forward thrust against the body by flattening the knee joint.
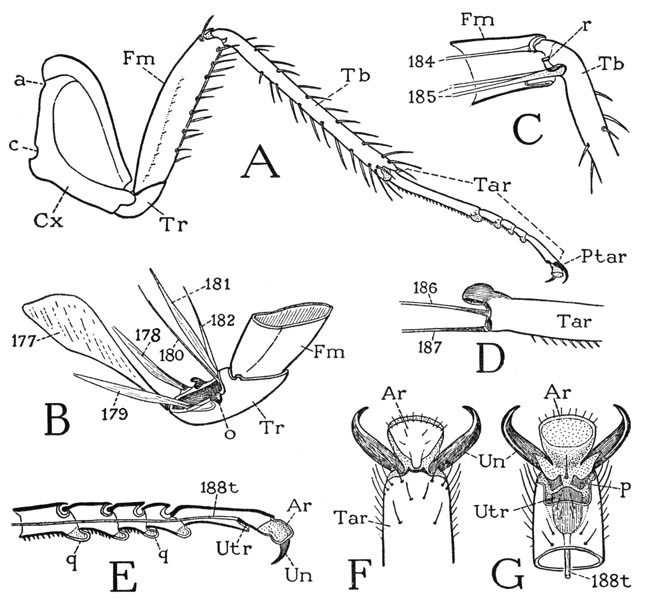
Fig. 83. Hexapoda—Pterygota. Periplaneta americana (L.). Structure of a leg.
A, left hind leg, anterior. B, trochanter and base of femur, with tendons of trochanteral muscles. C, the knee joint between femur and tibia. D, base of first tarsomere and its muscle tendons. E, tarsus and pretarsus, with tendon of pretarsal muscle. F, end of tarsus, and pretarsus, dorsal. G, same, ventral.
For explanation of lettering see pages 337–339.
It should be noted that the tendons of the trochanteral muscles are not attached literally on the wall of the trochanter but on the articular membrane close to the trochanteral margin; the same is true of most other insect tendons, otherwise they would be too rigid. The so-called “tendons” of arthropod muscles are really apodemes on which the muscle fibers are inserted; they are ingrowths of the body wall with a cuticular core. Muscles without tendons are inserted directly on the integument.
The femur is set closely on the oblique distal end of the trochanter (fig. 83 B, Fm) and has little movement other than a slight posterior flexion on the trochanter. A single flat muscle, the redactor femoris, arises in the trochanter and is inserted on the posterior margin of the femoral base. The obliquity of the trochanterofemoral joint in the dorsoventral plane of the leg allows the muscles of the trochanter to be effective in lifting or depressing the leg as a whole beyond the coxa. The femur is a long, thick segment; its size is due to the fact that it contains the large and important muscles of the tibia that move the part of the leg beyond the knee.
At the femorotibial joint the tibia has a dicondylic articulation on the end of the femur (fig. 83 C) with an anteroposterior axis, so that the tibial movement at the knee is one of levation and depression in the plane of the femur. The fibers of the tibial muscles completely fill the cavity of the femur, and their tendons of attachment on the tibia are easily seen. The tendon of the single levator muscle (184) pulls on the head of the tibia well above the articular axis (r); the tendons (185) of two depressor muscles arise close together from wide bases in the ventral articular membrane of the joint. An ample infolding of the ventral membrane allows the tibia to be closely flexed ventrally against the femur.
The tibiotarsal joint differs from the other joints of the leg in that the tarsus articulates on the tibia by a large dorsal knob on its base (fig. 83 D) that fits into an overhanging concavity on the end of the tibia. The tarsus thus has a free movement on the tibia, but it is flexible principally in a forward or ventral direction. It is provided with only two muscles, a depressor with its tendon (187) attached ventrally on the tarsus and a promotor (186) attached anteriorly.
The subsegments of the tarsus (fig. 83 E) are freely movable on each other, but they are not connected by muscles, which fact may be taken to mean that the tarsomeres are not true leg segments. The entire tarsus is traversed by the tendon of the pretarsal muscle (188t), the fibers of which arise in the tibia and the femur; the pull on the tendon very evidently must cause a ventral flexion of the tarsus when the latter is not held taut by the pretarsal claws.
The pretarsus projects from the end of the last tarsomere. The body of this apical segment of the leg is a soft, hollow lobe, termed the arolium (fig. 83, E, F, G, Ar); from the sides of its base arise the paired lateral claws, or ungues (Un). The dorsal wall of the arolium (F) contains a spatulate plate; the broad distal end (G) presents an oval disc with a smooth membranous surface devoid of setae. The arolium is an adhesive organ for holding to smooth surfaces that the claws cannot grasp. The large decurved claws are hollow outgrowths from the base of the arolium; dorsally they are articulated on the end of the tarsus (F). The claws of the insect foot are commonly called by entomologists “tarsal claws,” but they clearly belong to the pretarsus; their articulation on the tarsus means nothing more than does the articulation of the tarsus on the tibia. That the pretarsus is a true end segment of the insect leg corresponding with the clawlike apical segment, or dactylopodite, of a myriapod or crustacean leg is evident from the fact that it has its own muscle.
In the articular membrane at the base of the undersurface of the pretarsus (fig. 83 G) is a large plate, the unguitractor (Utr), on which is attached the tendon (188t) of the pretarsal muscle. Intervening between the unguitractor plate and the bases of the claws are two small accessory sclerites (p), termed auxilliae, and the proximal end of the unguitractor is deeply inserted into a pocket of the tarsus (E, G) to allow of its retraction. The pull of the pretarsal tendon on the unguitractor is directly transmitted to the bases of the claws, so that the latter are turned downward until their points hold on the irregularities of any ordinary surface. When the insect finds itself on a smooth, hard surface, however, the claws can offer no resistance, they then turn forward and the pull of the tendon is now transferred to the arolium, which is turned downward until its end disc is pressed flat against the surface. The arolium maintains its hold by simple adhesion; it leaves no smear when lifted or moved from one position to another, showing that there is no exuded substance to give it its adhesive properties. The same may be observed in flies and other insects walking on the undersurface of a glass slide.
The Wings
The insects are the only flying animals, except the winged creatures of fiction, that have not paid for their wings by the functional loss of a pair of legs. The wings of insects are new organs added to the primitive locomotor equipment; they are flat integumental folds of the back, strengthened by radiating cuticular thickenings that form hollow, branching ribs known as the veins. Blood circulates freely through each wing along definite courses, in general going outward from the body in the channels of the anterior veins and returning through those of the posterior veins, the transfer from one system to the other being by way of cross veins between the main branches (see Yeager and Hendrickson, 1934, and Clare and Tauber, 1939).
The mechanism of the wing movement is fairly simple, and the motor power in most insects is derived from muscles that probably were present in the wingless ancestors of the hexapods. The base of each wing is pivoted from below on the pleuron and is hinged to the edge of the tergum somewhat mesad of the pleural support. All that is needed to produce an up-and-down movement of the wings, therefore, is an alternate depression and elevation of the wingbearing tergum. The tergal movements in most insects are brought about by dorsoventral tergosternal muscles and longitudinal intertergal muscles. The dorsoventral muscles depress the tergum and cause the elevation of the wings; the longitudinal muscles arch the tergum upward and produce the downstroke of the wings. It must be noted, however, that the arching effect of the intertergal muscles on the back plates is dependent on a close union between the two wing-bearing terga and between the second wing-bearing tergum and the first abdominal tergum. Otherwise the force of the muscle contraction would be expended in pulling the consecutive terga together. In most flying insects these two sets of indirect wing muscles are greatly enlarged in each of the wing-bearing segments. Since this mechanism of wing movement is as fully developed in the Ephemeroptera (Diirken, 1907) and Plecoptera (Grandi, 1948,1949) as in the higher insects, it would seem probable that it gave the first up-and-down movement to the primitive wing folds when the latter became flexible on the body. In order that the wings may act as propellers, however, it is necessary that each wing should have a torsion on its long axis. Anterior and posterior movements of deflection are produced by muscles inserted on the basalar and subalar sclerites of the pleuron closely associated with the wing base respectively before and behind the pleural fulcrum.
The wings of most insects have, in addition to the flight mechanism, a mechanism of flexion and extension in a horizontal plane, by which the wings when not in use are folded posteriorly over the back and brought forward preliminary to flight. A piece of stiff paper attached by one edge to a support cannot be turned horizontally without crumpling; conversely, the wing has a crumpling device in its base which automatically turns the wing posteriorly in a horizontal plane. If the wing is broad, the same mechanism may cause it to fold lengthwise on itself. The flexing apparatus is operated by a special flexor muscle; extension probably results from the pull of the basalar muscle on the anterior angle of the wing base, but the subalar muscle must contribute to the extension movement by restoring the crumpled area of the wing base to a flat condition.
The dragonflies appear to have a wing mechanism different from that of other flying insects, inasmuch as their wings are moved by muscles attached directly on the wing bases laterad and mesad of the pleural fulcra. In a study of the dragonfly muscles it has been shown by Clark (1940), however, that nearly all the wing muscles can be identified with muscles of ordinary flying insects, suggesting that the flight mechanism of the dragonflies is merely a specialization of the primitive musculature and not something radically different from that of other flying insects. Dragonflies do not flex the wings, and consequently all the muscles associated with the wings can be used for flight. The dragonfly’s mechanism of flight appears to be the simplest and most efficient way of moving the wings, but it could not be derived so directly from the structure of a nonflying ancestor as that of other insects. The thorax of a dragonfly is highly specialized to accommodate its wing mechanism (see Sargent, 1937, 1951). Though the dragonflies are among the oldest of known insects, being fully developed in Carboniferous times, they are not primitive insects and are not ancestral to any of the other insect orders.
By contrast with the dragonflies, the cockroaches, the mantids, and the termites have a well-developed wing-flexor mechanism, but a very poorly developed mechanism of flight. Since the mayflies have the usual flight muscles of other insects, but no wing-flexing mechanism, it would seem that flexion of the wings was a secondary innovation. It follows, then, that the cockroaches, mantids, and termites must be descended from insects that had a fully developed flight mechanism; their diminished power of flight is due to a great reduction or absence of the dorsoventral and longitudinal dorsal muscles of the wing-bearing segments. Still these insects are able to fly, and in the following study of the wing mechanism of Periplaneta we shall attempt to understand how they do it.
The two pairs of wings of Periplaneta are very different in size and form. The large hind wings undoubtedly are the principal organs of flight, the forewings, or tegmina, serving as coverings over the more delicate hind wings when the latter are flexed and folded over the back. The tegmina show by their venation that they are wings of the same type of structure as the hind wings, but the wing structure will be most easily understood from a study of one of the metathoracic wings.
A hind wing of the cockroach when spread out flat (fig. 84 A) has the appearance of two wings stuck together along a line of folding (vf). The part before the fold is the wing area principally effective in flight and may be termed the remigium (Rm); the large, fanlike expansion behind the fold is sometimes termed the neala, implying that it is a secondary enlargement of the wing, but its shape suggests the name vannus (Vn), which is Latin for “fan.” The narrow area mesad of the vannus, separated from the latter by a second fold (jf), is the jugal region. The venation is entirely different in the two major parts of the wing, but the principal veins of the remigial region are those characteristic of the wings of insects in general.
The first two veins are the costa (fig. 84 A, C) and the subcosta (Sc), which in the cockroach are unbranched and together form a strong marginal thickening along the basal half of the wing. The third vein is the radius (R), which has two main stems and numerous terminal branches. The fourth vein is the media (M) with only a few terminal branches; in the adult wing the media is united basally with the radius, but the corresponding trachea in a nymphal wing has an independent origin (see fig. 117 in Comstock, 1918). The next vein is the cubitus (Cu), and is the most profusely branched vein of the wing. Behind the cubitus are three long, slender, unbranched veins termed the “plical veins.” Only the third one, however, is a true plical vein, since it lies in the vannal fold; it is sometimes called the vena dividens. The other two may be designated simply postcubitals (D, Pcu), though in most insects there is only one postcubital in front of the fold. The veins of the fan are the vannal veins, and those of the jugal region the jugal veins. The vannal veins all arise from an arcuate vein in the base of the fan and thus form a very distinct group of veins separated from those of the remigium by the vannal fold. The postcubitals and the vannal veins together are the anal veins of the Comstock-Needham system of vein nomenclature, but in the nymphal wing of the cockroach a postcubital trachea is independent of the vannal group, and in the mechanism of the adult wing the postcubitals belong to the remigium. Details of the branching of the veins will be found to differ somewhat in different specimens, and between the main branches are small secondary veins and numerous cross veins not shown in the figures.
The wing veins have definite relations to small sclerites, the axillaries, in the base of the wing (fig. 84 D), which determine the wing movements of extension, flexion, and folding. There are three principal axillaries, generally termed the first axillary (1Ax), the second axillary (2Ax), and the third axillary (3Ax), but a median plate (m) associated with an arm of the third axillary is also an important element of the wing-base mechanism.
The first axillary (fig. 84 B, D, 1Ax) is a triangular sclerite produced anteriorly in a long neck that rests on the anterior wing process of the notum and abuts against the head of the subcostal vein (Sc). By its mesal margin the body of the first axillary is closely hinged to the edge of the notum and is thus the principal hinge plate of the wing. The second axillary (2Ax) is an elongate sclerite lying just laterad of the first (D); anteriorly it is connected with the head of the radial vein (R) and posteriorly with the third axillary, but it has a long ventral process (B, g) closely attached to the large subalar sclerite (Sa) in the pleuroalar membrane below the wing. The body of the second axillary rests on the pleural wing process, and this axillary is therefore the pivotal plate of the wing base. The third axillary (B, D, 3Ax) has a complex structure; mesally it presents a large, concave disc (B, h) on which is attached a muscle from the pleural apodeme; laterally it is extended in a posterior arm (k) associated with the bases of the cubital and postcubital veins of the wing (D), anteriorly it gives off a larger arm (l) closely hinged to the edge of the median plate (m) of the wing base. The crescentshaped muscle disc (B, h) articulates by its anterior horn (i) with the posterior end of the dorsal part of the second axillary (f), as seen at D, and by its posterior horn (B, j) with the posterior wing process of the notum (D). Ventrally, the third axillary is strongly connected by a stalklike arm (B, n) with the posterior part of the subalar plate (Sa).
The third axillary, being the only plate of the wing base on which a muscle is attached, is the active agent for the flexing and folding of the wing. The downward pull of the pleural muscle on the muscle disc of this axillary revolves the axillary on its articular points (B, i, j) and turns the lateral arms (k, l) upward. The posterior arm (k) pulls directly on the bases of the cubital and postcubital veins (D), the anterior arm (l) forms a sharp upward fold with the median plate (m); the whole remigium in consequence swings posteriorly on the hinge of the subcosta with the first axillary, which latter turns vertically on the edge of the notum. As the remigium is thus turned posteriorly and mesally by the revolution of the third axillary, the wing is folded along the line of the vannal plica and the hinge between l and m, so that the remigium goes over the vannus, and the latter is turned upside down beneath it (C), until finally the folded wing takes a longitudinal position over the back of the abdomen.
Extension of the wing is perhaps initiated by the tension of the basalar muscle transmitted through the basalar sclerite to the humeral angle of the wing, but an extensor action of the basalare is not easy to demonstrate on a dead cockroach. It seems probable, however, that, since the third axillary is strongly connected with the subalar plate, the pull of the subalar muscle would restore this axillary to its position in the expanded wing and thus automatically bring about an extension of the wing.
Fig. 84. Hexapoda—Pterygota. Periplaneta americana (L.). The wing.
A, right hind wing fully spread out. B, axillary sclerites, subalar sclerite, and median plate of wing base, dorsal. C, section of folded right wing. D, base of right wing and attachment on metanotum.
d, alar sclerite of metanotum; f, dorsal process of second axillary; g, ventral process of second axillary connecting with subalar sclerite; h, muscle disc of third axillary; i, articular point of third axillary with f of second axillary; j, articular point of third axillary with posterior notal wing process; k, posterior lateral arm of third axillary; l, anterior lateral arm of third axillary; m, median plate of wing base; n, ventral subalar process of third axillary. For explanation of other lettering see pages 337–339.
The flight mechanism of the cockroach is not well understood. Most winged cockroaches fly; some are fairly good flyers, and even Periplaneta is known to fly on occasions, but the cockroach ordinarily puts its trust in the locomotor efficiency of its legs rather than in its wings. The dorsoventral muscles that in most insects produce the upstroke of the wings by depressing the notum are entirely absent in the cockroach. Three pairs of oblique dorsal muscles from the middle of the metanotum to the first abdominal tergum can hardly be supposed to have a depressor action on the metanotum, but a pair of large flat tergopleural muscles attached on the lateral edges of the notum should pull down on the notal margins and thus serve to elevate the wings on the pleural fulcra. Also, there are attached on the notum numerous muscles of the legs that could effect an elevation of the wings.
The mechanism of the important downstroke of the wings, on the other hand, is difficult to understand, considering the small size of the dorsal intertergal muscles and the fact that the wing-bearing nota of the cockroach are connected by intervening membranes, so that it would seem whatever force the dorsal muscles may exert would be expended in merely pulling the back plates together. As we have already noted, however, both the second axillary and the third axillary of the wing base are strongly connected with the subalar sclerite, and the latter gives attachment to a huge muscle from the meron of the coxa. This muscle, therefore, must have some important action on the wing. Probably in most insects the subalar muscle contributes to the downstroke of the wings. In the cockroach the subalar muscle has a very oblique, almost horizontal, position, and yet it appears to be the only muscle capable of a depressor action on the wing. The thoracic musculature of strong-flying cockroaches, such as Parcoblatta, appears to be the same as that of Periplaneta.
The thoracic musculature of the mantis, as described by Levereault (1938) for Stagmomantis Carolina, is very similar to that of the cockroach, except that in the mantis there are no dorsal longitudinal muscles in either of the winged segments, and there is present in the mesothorax a pair of slender tergosternal muscles. Yet the mantis is a relatively strong flyer, though its progress on the wing is slow and is not sustained for long distances. The downstroke of the wings of the mantis Levereault suggests may be produced by certain muscles that pull backward on the basalar sclerites and thus presumably arch “the tergum over the wing fulcra,” but Levereault does not mention the subalar muscle as a possible wing depressor, though this muscle is strongly developed in the mantis and is less oblique than in the cockroach. In the winged termites the thoracic musculature, as described by Fuller (1924), is again much the same as that of the cockroach, though longitudinal dorsal muscles appear to be entirely absent, and in the mesothorax there is a small anterior tergosternal muscle. Fuller does not discuss the wing mechanism of the termite, but he notes that the third axillary is closely associated with the subalar sclerite, and that on the latter is attached a huge muscle from the meron of the coxa.
The mesothoracic wings, or tegmina, of Periplaneta are a little longer and not so broad as the outspread hind wings, but they have the same general plan of structure and pattern of venation. When the tegmen is flexed, however, there is no folding between the remigium and the vannus; but the triangular jugal region folds completely beneath the vannus, allowing the rest of the tegmen to take a flat position over the folded hind wing (fig. 77). The parts of the axillary mechanism that produce the folding of the hind wing, such as the lateral arms of the third axillary and the median plate of the wing base, are consequently much reduced in the tegmen, but the connection of the third axillary with the subalare is even stronger than in the metathorax. When the wings are folded, the left tegmen partly overlaps the right; the same is true in the mantids and the termites.
The Abdomen
The abdomen of the cockroach consists of ten segments with distinct dorsal plates and includes a group of terminal lobes around the anus that probably represent an eleventh segment. Inasmuch as entomologists commonly designate the segments of the insect abdomen numerically as abdominal segments distinct from the thoracic segments, the abdominal segments will be numbered in the following descriptions as segments 1–11, and not as body segments 4–14.
General Structure of the Abdomen— The abdomen of Periplaneta (fig. 85 A) is broad and somewhat flattened anteriorly, narrowed and more rounded posteriorly (D). On the back there are ten fully formed tergal plates, but the ninth tergum in the male (B, 9T) and both the eighth and the ninth terga in the female (C) are narrow and may be largely overlapped by the tergum in front. The tenth tergum is a terminal shield-shaped plate in each sex (B, C, 10T) with a deep posterior emargination; at the sides of its base arise the cerci. On the venter there are nine sternal plates in the male (A) and only seven in the female (C). The first sternum is reduced to a small median plate (A, 1S) in an otherwise membranous ventral part of its segment. The ninth sternum of the male carries a pair of slender styli (A, Sty), the only styli present in the cockroach. The seventh sternum of the female (C, 7S) supports a pair of large, oval apical lobes (otL) turned upward against the anal region. The rounded shape which these lobes give to the end of the abdomen at once distinguishes the female of Periplaneta from the male. In the male (A) a group of genital structures (Phl) projects from the end of the abdomen.
The lateral margins of the tergal and sternal plates are closely adjacent, but they are separated on the sides by infoldings of the integument between them (fig. 85 D) that allow a dorsoventral expansion and contraction of the abdomen. When the tergum and the sternum of a segment are pulled apart (E), it is seen that the walls of the infold on each side contain small sclerites, two adjoining the tergum, which may be termed laterotergites (ltg), and one closely connected with the sternum, which may be designated a laterosternite (lst), The laterotergites of each side include a small anterior plate and a long, narrow posterior plate, with a spiracle (Sp) between them. Some writers regard these lateral sclerites of the abdomen as “pleurites,” perhaps because the spiracles have a pleural position on the thorax, but in most adult insects the abdominal spiracles are in the lateral parts of the terga.
Though the infoldings along the margins of the abdominal segments allow of a dorsoventral expansion of the abdomen, the cockroach makes no perceptible abdominal movements of respiration, such as those of most other orthopteroid insects. At times, however, there may be observed a lengthwise protraction and retraction of the segments on each other.
Fig. 85. Hexapoda—Pterygota. Periplaneta americana (L.). The abdomen and the spiracles.
A, abdomen of a male, ventral. B, end of male abdomen, dorsal. C, end of female abdomen, left side. D, cross section of posterior part of abdomen. E, adjacent parts of tergum and sternum of fifth abdominal segment separated to show intervening laterotergites and laterosternite, left side. F, terminal part of abdomen, ventral. G, first thoracic spiracle, left. H, second thoracic spiracle, left. I, laterotergites and spiracle of an abdominal segment, left. J, inner view of an abdominal spiracle, with trachea removed. K, lateral view of a left abdominal spiracle with stump of trachea attached.
For explanation of lettering see pages 337–339.
The infolded membrane between the fifth and sixth abdominal terga of the male of Periplaneta forms a deep pocket, and at the bottom of it may be seen two small, transverse, slitlike pouches with thick, soft walls. These apparently glandular organs probably correspond with the more highly developed dorsal glands of some other cockroaches (see Oettinger, 1906), which are said to produce a secretion sought after by the female at the time of mating.
Beneath the tenth tergum is the short, conical eleventh segment of the cockroach abdomen (fig. 85 F), known as the proctiger because it contains the anus (An), which latter is surrounded by four small apical lobes. In the lateral walls of the segment are a pair of thick sclerites, the paraprocts (Papt), the setigerous ends of which form the lateral anal lobes. Above the anus is a short, rounded lobe, the epiproct (Eppt), and below it the ventral wall of the segment is produced into a small hypoproct (Hypt). At the bases of the paraprocts arise the cerci (Cer), which in the cockroach evidently belong to the eleventh abdominal segment, as they do in the Thysanura (figs. 75 J; 76 L), though in many insects in which the eleventh segment is reduced or obliterated the cerci are taken over by the tenth, and in the cockroach the cerci have basal articulations on the tenth tergum (C).
The Cerci— The long, tapering cerci are movable by muscles attached on their bases, and each appendage is divided into about 15 short rings, so that it is freely flexible and not easily broken. The somewhat flattened upper surface is smooth except for the presence of minute hairs; the convex undersurface, however, is covered with longer, slender hairs. Each cercus is traversed by a large nerve from the last abdominal ganglion. It has been shown by Pumphrey and Rawdon-Smith (1936) that the cerci of Periplaneta and Gryllus bear sense organs receptive to sound. On applying to a cereal nerve fine electrodes connected with an amplifier and a recording apparatus, these investigators demonstrated that exposure of the cerci to sound from a loud speaker produces an electric response in the nerve that varies with the pitch of the sound. Covering the ventral hairs of the cerci of the cockroach with vaseline abolished the nerve response, indicating that these hairs are the sound receptors. A live cockroach usually carries its cerci fully exposed in an erect position, and Pumphrey and Rawdon-Smith add that it seems probable that the function of the cercus in the cockroach “as a wind-gauge may be equal in importance to its function as an acoustic organ.”
The Abdominal Spiracles— The spiracles of the abdomen are much smaller than those of the thorax, and each presents externally merely a vertical slit in a slightly protruding, soft, oval marginal rim. The spiracles of the first pair lie dorsally in small membranous areas at the sides of the first abdominal tergum. The other seven are situated between the two laterotergal plates (fig. 85 E, I, Sp) of their respective segments. The spiracles of the second segment are exposed ventrally (A) but the others are concealed by the overlapping posterior angles of the preceding sterna.
The simple opening of an abdominal spiracle is directed posteriorly and leads into a cup-shaped atrium (fig. 85 K, Atr); the atrium opens through a short membranous ring into a single, large tracheal trunk (Tra). The walls of the atrium are hexagonally reticulated, but the inner margins are thickened. From the marginal thickening on the outer (anterior) side of the atrial wall there projects forward and downward a tapering, fingerlike process, and from the inner (posterior) wall a shorter and wider process. Between these two processes is stretched an occlusor muscle (J, ocmcl), and on the end of the outer process is attached an opening, or dilator, muscle (dlmcl) that arises on the small laterotergal plate before the spiracle. The abdominal spiracles are thus closed and opened by a valvelike mechanism that controls the atrial entrance into the trachea, and in this way they differ mechanically from the thoracic spiracles that are closed by bringing the outer lips together.
The Male Genitalia— At the end of the abdomen of a male Periplaneta (fig. 85 A) are seen a number of prongs, hooks, and lobes projecting from beneath the tenth tergum (B) and above the ninth sternum (A). These structures are the external genital organs of the male. By removing the tenth tergum (fig. 86 A) it will be seen that the various parts pertain to three separate organs, two of which are dorsal, one right (rPhm), the other left (lPhm), and the third ventral (vPhm). The complex genitalia of the male cockroach serve principally for clasping and holding the female during copulation; they do not form an intromittent organ for the injection of sperm. A spermatophore is introduced into the genital chamber of the female and attached to the mouth of the spermatheca. The term “penis valves” often given to the genital organs of the male cockroach is, therefore, inappropriate; if the group of organs is termed the phallus, the three components are phallomeres (i.e., phallic parts).
The entire phallic complex of Periplaneta can be traced back in its development, as shown by Qadri (1940), to a single pair of small primary phallic lobes that are present on the venter of the tenth abdominal segment in first and second instar nymphs (fig. 86 F, phL). Between the lobes an ectodermal ingrowth forms the beginning of the ejaculatory exit duct, which later unites with the terminal ampullae (Amp) of the vasa deferentia (Vd) and thus becomes the outlet of the mesodermal reproductive organs. In the third instar the primary phallic lobes have increased in size and each has divided into two parts (G). Those of the right lobe, according to Qadri, become the right phallomere and the ventral phallomere of the adult; the parts of the left lobe (lPhm), however, are not completely separated and together form the duplex left phallomere of the adult. The gonopore (Gpr), or opening of the ejaculatory duct, lies between the two dorsal phallomeres and above the base of the ventral phallomere. The early developmental history of the genitalia in all the orthopteroid insects, as shown by Qadri, is similar to that in Periplaneta, but the final development into the adult organ may be quite different in the several orders.
From the work of a number of investigators it is now known that in most of the insects above the Orthoptera the external genital organs of the male take their origin, as in the Orthoptera, from a pair of phallic lobes at the sides of an ingrowth that will become the ejaculatory duct. Each lobe, moreover, divides into two branches, but one branch, which may be termed the mesomere, is mesal, and the other, known as the paramere, is lateral. From here on the development is quite different from that in the Orthoptera. The meso-meres unite to form a tube, the lumen of which is continuous with that of the ejaculatory duct, and the structure so formed is the intro-mittent organ known as the aedeagus (or the mesosome in Diptera). The parameres and the aedeagus may retain a common base, or the parameres may become disconnected from the aedeagus and form independent clasping organs. On the other hand, genital claspers may be the styli of the ninth segment, as they certainly are in the Ephemer-optera and perhaps in some other insects, or again they may be the cerci of the eleventh segment. Finally, the copulatory apparatus in many insects becomes complicated by the development of various secondary accessory structures. Since the aedeagus and the parameres have a common origin from the primary genital lobes, they are both phallic organs; other structures of a different origin may be grouped as periphallic organs. Because of their endless diversity of structure, the male genitalia of insects furnish excellent characters for the separation of species, but, in spite of the large amount of work that has been done on them, the homologies of their parts and the primary nature of the organs are still far from certain. Some writers have regarded the phallic lobes as representing a pair of segmental limb appendages, but the supposed homology with primitive abdominal legs has not been demonstrated. There is likewise no specific evidence that the lobes represent paired penes, such as those of the Ephemeroptera, since at no time in their known development are they traversed by exit ducts.
Fig. 86. Hexapoda—Pterygota. Periplaneta americana (L.). The male genitalia and the spermatophore.
A, genital organs as seen from above, projecting from anterior wall of genital chamber. B, right phallomere, dorsal, ventral parts exposed by removal of wall of genital chamber, cut off at z. C, right phallomere, dorsal, turned forward to expose ventral plates (opl), which are artificially opened. D, left phallomere and phallic gland, dorsal. E, ventral phallomere and ejaculatory duct, dorsal. F, primary genital lobes of second instar (from Qadri, 1940). G, genitalia at later stage, showing division of primary lobes into four parts. H, accessory glands and seminal vesicles on anterior end of ejaculatory duct. I, spermatophore, diameter 1.3 mm. (from Gupta, 1947a). J, section of spermatophore (from Gupta, 1947a).
For explanation of lettering see pages 337–339.
The right phallomere of Periplaneta, as seen from above in the undisturbed condition beneath the base of the tenth tergum (fig. 86 A, rPhm), presents a strongly sclerotized plate extending to the left and ending in a pair of sharp prongs, and on the right is a small accessory hook. When the membranous body wall at its base is removed, however, other parts are exposed (B), which are best examined by turning the pronged plate upward and forward (C). There will now be exposed a deep cavity in the ventral part of the phallomere, open posteriorly, between two strong horizontal plates (opl) resembling the valves of a clam shell. In the figure the plates are artificially opened; they are hinged to each other on the left by incurved, fingerlike processes, and on the right they are articulated on the ends of a supporting sclerite. The two plates closed upon each other, as seen when exposed from above, are shown at B.
The left phallomere (fig. 86 D) is a complex of several parts with a common base. On the extreme left is a long, slender arm with a strongly curved terminal hook, to the right of it is a second shorter and thicker arm having a transverse, hammer-head enlargement at the end, and from the mesal part of the phallomere there project several soft lobes, one bearing a small hook. Through the left phallomere opens a long, flat, feather-shaped phallic gland (phGld), the duct of which traverses the body of the phallomere to open distally near the right side (glO) between the edge of a mesal plate and the base of the hook-bearing lobe.
The ventral phallomere is a rather large, simple lobe with a rounded free end projecting to the right from beneath the right phallomere (fig. 86 A, vPhm). Its ventral surface is a smooth plate (E), but its dorsal integument is soft and is produced to the left in a large rounded lobe. The ejaculatory duct (Dej) opens from the left (Gpr) into a depression at the base of the lobe.
The Spermatophore— The spermatophore of Periplaneta americana is shown by Gupta (1947a) to be a pear-shaped capsule 1.3 mm. in diameter (fig. 86 I). The wall of the completed spermatophore is composed of three layers of noncellular secretion material (J). The innermost wall, as described by Gupta, is formed in the enlarged upper end of the ejaculatory duct (H, Dej) by secretion from the long lateral accessory glands opening into the duct. The capsule is at first open at its upper end and receives the spermatozoa from the seminal vesicles (Vsm) together with a liquid from the small median tubules of the accessory glands. The inseminated capsule is then closed except for an opening at its larger upper end, and as it passes down the ejaculatory duct it receives the second layer of its wall from the epithelial cells of the duct. Finally, it is attached during mating to the spermathecal papilla of the female, and a secretion from the phallic gland is now poured over it, which hardens to form the outer coating.
The Female Genitalia— The external genital structures of the female cockroach are entirely concealed within a large cavity in the end of the abdomen closed posteriorly by the upturned apical lobes of the seventh abdominal sternum. The cavity contains the ovipositor and the opening of the oviduct; if the female is carrying an egg packet, or ootheca, it is held in the posterior space between the sternal lobes. In a longitudinal section of the abdomen, as shown diagrammatically at A of figure 87, it will be seen that the short ovipositor (Ovp) arises from the dorsal wall of the cavity and that the oviduct (Ovd) opens on the floor of a flattened anterior pocket (GC) above a transverse fold of the ventral wall reflected back from the end of the seventh sternum (7S). Since this pocket lies proximal to the base of the ovipositor, it may be regarded as the true genital chamber of the female cockroach corresponding with that of other insects having the ovipositor fully exposed. The posterior part of the main cavity is the oothecal chamber (otC), termed also the vestibulum, a secondary enclosure within the lobes of the seventh sternum (otL), in which the ootheca is formed. In the dorsal wall of the genital chamber is the aperture of the spermatheca (Spt), and just behind the base of the ovipositor is the opening of the female accessory glands (AcGlds). The eggs issuing from the oviduct are therefore inseminated in the genital chamber and guided back by the ovipositor into the oothecal chamber, where they are covered by the secretion from the accessory glands, which hardens to form the shell-like ootheca.
The ovipositor and associated structures are best seen in a ventral view (fig. 87 C) as exposed by removal of the seventh sternum and its apical lobes. In the figure, the floor of the genital chamber containing the gonopore (Gpr) has been turned forward in the plane of the dorsal wall, as indicated by the arrows. The gonopore is a long median slit opening from a wide but shallow pouch that represents the unpaired oviduct. The lips of the aperture are strengthened by a pair of plates (a, a), at the sides of which are two elongate plates (b, b) that, in the normal position, diverge posteriorly at the sides of the ovipositor. In the roof of the genital chamber are two large lateral plates (c, c) and a small median plate (d). The median plate has a short anterior neck that ends in a small membranous lobe known as the spermathecal papilla, shown more enlarged at F, which contains the aperture of the spermatheca (C, sptO). The spermatheca (G) consists of two tubes, one longer than the other, with a short common outlet duct. The distal part of the longer tube becomes gradually thickened toward the end and is thrown into various loops; the shorter tube has an apical enlargement at the end of a long slender duct. In the wide part of each tube the axial duct is conspicuous by its dark color; numerous fine striations radiating from it are intracellular ductules of the thick glandular epithelium. Surrounding the epithelium, within an outer tunic, according to Gupta (1948), is a layer of muscle fibers. Each spermathecal tube of the inseminated female of Periplaneta is said by Gupta to contain spermatozoa.
The general anatomy and the musculature of the genital region of the adult female cockroach, as shown by Ford (1923), suggest that the plates on the floor of the genital chamber at the sides of the gonopore (fig. 87 C, a, b) represent the eighth abdominal sternum deeply inflected above the seventh sternum. However, it is said by Gupta (1948) and by earlier students of the development of the genital parts of the cockroach, that the oviduct is formed as an ingrowth in the intersegmental membrane between the seventh and the eighth sterna, and that the female gonopore of the cockroach thus retains a primitive position behind the seventh sternum, while in most of the higher insects it has secondarily come to lie behind the eighth. According to Gupta, the plates in the dorsal wall of the genital chamber are derived from the eighth sternum, but, if so, it is a very unusual thing for the spermatheca to open on the eighth sternum.
Fig. 87. Hexapoda—Pterygota. Periplaneta americana (L.). The female genitalia.
A, longitudinal section of end of abdomen, showing the oothecal chamber (otC) containing the ovipositor, and the genital chamber (GC) into which the oviduct opens ventrally and the spermatheca dorsally. B, seventh abdominal sternum and its apical oothecal lobes (otL) enclosing the oothecal chamber, dorsal. C, the ovipositor, and plates of dorsal wall of genital chamber surrounding orifice of spermatheca (sptO), ventral, with floor of genital chamber turned forward as indicated by arrows. D, second and third valvulae of ovipositor, ventral. E, cross section of oothecal chamber and enclosing lobes, posterior. F, spermathecal papilla, ventral. G, spermatheca.
a, b, plates of ventral wall of genital chamber; c, d, plates of dorsal wall of genital chamber; e, basal plates of second and third valvulae; f, g, connections of ovipositor with eighth and ninth terga. For explanation of other lettering see pages 337–339.
The short ovipositor of the cockroach consists of the same three pairs of elongate processes that form the ovipositor of most insects. The processes are known as valvulae and are distinguished as the first, second, and third, or as the ventral, intermediate, and dorsal valvulae. In Periplaneta the first valvulae (fig. 87 C, 1Vl) have an independent origin anterior to the other valvulae behind the lateral plates (c, c) of the dorsal wall of the genital chamber. They are long arms converging posteriorly beneath the second and third valvulae; the widened base of each one is connected by a very slender bar (f) in the lateral wall of the oothecal chamber with the outer end of the eighth tergum (8T), showing that the first valvulae belong to the eighth abdominal segment. The second and third valvulae (C, D, 2Vl, 3Vl) have a common base formed of two large plates (e, e). The second, or intermediate, valvulae are slender, tapering arms (D, 2Vl) arising from the basal plates. The third valvulae (3Vl) are direct continuations from the basal plates; they are longer and thicker than the second valvulae, with concave undersurfaces and the ends turned downward over the tips of the second valvulae. The second and third valvulae belong to the ninth abdominal segment, and each basal plate is connected by a lateral extension with the expanded anterior end of a slender bar (C, g) from the ninth tergum (9T). The anterior end of this bar, however, is attached also to the base of the first valvula.
The ovipositor of most insects has a well-developed mechanism by which the prongs slide back and forth on each other, but no such mechanism is evident from the structure of the cockroach ovipositor, and it is therefore difficult to identify its basal parts with those of a more typical egg-laying organ. The ovipositor of the cockroach serves merely to conduct the eggs back to the oothecal chamber, and, because of its concealment, whatever activity it may have has not been observed.
The female genital accessory glands of Periplaneta, two in number with a common opening, are long, dichotomously branched tubes, disposed in a great tangled mass in the abdominal haemocoele beneath the rectum. The aperture of the glands lies between the bases of the second valvulae (fig. 86 C, D, acgldO). The secretion furnishes the material for the construction of the ootheca.
The oothecal chamber is enclosed beyond the ovipositor between the apical lobes of the seventh abdominal sternum (fig. 87 B, otC). The outer surfaces and the incurved dorsal parts of the lobes (B, E, otL) are strongly sclerotized plates, but the inner walls are soft and covered by a delicate cuticle. The upper margin of each lobe is produced into a thick dorsal fold (E, df). The lower edges of the two lobes are connected by a bridge of soft integument (vw) forming the floor of the oothecal chamber (otC), which is ordinarily deeply inflected but allows of a great lateral expansion of the chamber when an egg capsule is being formed within the latter. When the oothecal chamber is empty, the dorsal folds of the lobes may be variously disposed, but when an ootheca is being formed they stand up and embrace the upper part of the capsule, which is molded between them. A more detailed account of the structure of the posterior part of the female abdomen of Periplaneta is given in a recent paper by Brunet (1951).
The relation of the male and female genital structures of Periplaneta during mating has been described by Gupta (1947b), who shows that the various parts of the male phallic apparatus have specific copulatory functions. The hooked arm of the left phallomere (fig. 86 D) pulls down on the apical lobes of the seventh sternum of the female abdomen and opens the genital cavity. The end of the hammer-headed arm is then thrust into the opening of the oviduct, turned transversely, and thus anchored beneath the gonopore plates (fig. 87 C, a, a). The “clam-shell” plates of the right phallomere, called the opposing lobes by Gupta (fig. 86 C, opl), grip the distal ends of the anterior valvulae of the ovipositor, the prongs of the dorsal plate grasp the base of the right first valvula, and the curved spine on the right holds the base of the left valvula. The spermatophore is then discharged upon the dorsal surface of the ventral phallomere of the male and is attached to the spermathecal papilla of the female.
The Ootheca— The ootheca of Periplaneta is a hard-walled, dark reddish-brown, purse-shaped capsule (fig. 88 C), about 9 mm. in length, having a straight-edged crest along its upper side with a finely serrate margin. It is somewhat compressed from side to side and is pear-shaped in cross section (D). Within the capsule is a double row of eggs, seven or eight on each side, set vertically with the ventral surfaces of the contained embryos toward each other, and the head ends upward. Lines on the outer surface of the ootheca mark the position of the eggs, which are separated by slight internal ridges. The capsule is closed along the margin of the crest, but a gentle manipulation reveals a median cleft where the two edges come together (D), and, when the young cockroaches are ready to emerge, the ootheca normally opens along this line. In the formative stage of the ootheca, however, the serrated upper edges of the lateral walls are firmly attached to each other, and there is no natural opening here into the interior of the capsule. The structure of the ootheca of different species of cockroaches is described by Lawson (1951).
Fig. 88. Hexapoda—Pterygota. The ootheca and newly hatched young of a cockroach.
A, Periplaneta americana (L.), end of abdomen of female carrying a fully formed ootheca, lateral. B, same, ventral. C, the ootheca. D, cross section of ootheca showing position of eggs in two rows. E, Blattella germanica (L.), newly hatched young beginning to emerge from embryonic cuticle (from Pettit, 1940).
As already noted, the egg case is formed within the oothecal chamber of the female from the secretion of the accessory glands, which hardens as it is poured over the issuing eggs. Since the eggs in the ovary have the head ends forward, the ovipositor somehow sets them on end in the two opposing rows. At first the lobes of the seventh sternum stand erect and close the distal end of the oothecal chamber, but as the number of eggs increases the ootheca protrudes from the body of the female, while the lobes are depressed and spread apart. The fully formed capsule is held only by its anterior end in the grasp of the tenth tergum above, the oothecal lobes on the sides, and the everted soft ventral wall of the oothecal chamber below (fig. 88 A, B), until finally it is dropped and left for the embryos to mature and the young cockroaches to break out.
The texture of the ootheca might suggest that the cockroach egg capsule is a chitinous structure, but the secretion of the accessory glands is nonchitinous and is of a different composition in the two glands. It has been shown by Pryor (1940) in Blatta orientalis that the left gland secretes a water-soluble protein, while the other produces a dihydroxyphenol. The phenol is oxidized in air to a quinone that combines with the protein, which then becomes a scleroprotein resistant to most chemical reagents and enzymes.
The chorion of the eggs within the ootheca is a delicate membrane. On hatching, the cleft along the upper edge of the capsule opens, probably by the activity of the young cockroaches; the escape of the young of Blattella germanica has been interestingly described and illustrated by Pettit (1940). When the ootheca opens, the young cockroaches thrust out their heads and begin to swallow minute bubbles of air, which accumulate in the alimentary canal until the insects are almost doubled in size and are forced partly out of the capsule; they finally liberate themselves by their own activities. Each individual, however, is still enclosed in a thin, baglike embryonic cuticle, but the envelope soon splits along the back (fig. 88 E) and the free nymph runs off. At first the young cockroach is almost transparent because of the contained air, but on release of the air the normal size is restored, and a dark pigmentation soon follows.
Female cockroaches often carry the ootheca projecting from the abdomen a varying length of time before it is dropped. From this habit of carrying an egg-case full of embryos, it is only an evolutionary step to retaining the case in the body until the eggs hatch, and, in fact, there are various genera of so-called “viviparous” cockroaches. The structural modifications of the female organs that provide for the retention of the ootheca have been described in Diploptera dytiscoides by Hagan (1941,1951). In the female of Periplaneta it is to be noted that there is a small anterior pocket of the oothecal chamber below the overhanging posterior edge of the floor of the genital chamber (fig. 87 A, h). In Diploptera and other viviparous species this pocket is enlarged into a huge sack projecting forward in the abdomen as far as the thorax, usually on the left side. Within this pouch the ootheca is lodged until the hatching of the eggs. The ootheca of Diploptera, according to Hagan, is a thin membrane, which only partly envelops the egg mass, and it never turns brown. The left accessory gland, the protein-secreting gland of Blatta, Hagan says, continues its secretory activity after the formation of the ootheca and “may be a nutrient organ for the embryos.”
Since the ootheca is built up from behind forward in the oothecal chamber, it remains to be explained how it gets into the brood pouch. In the viviparous Gomphadorhina laevigata, Chopard (1950) has observed that the forming ootheca is first slowly extruded in the usual manner from the body of the female, but that, when it is held only by its extremity, a reverse movement takes place by which the capsule is slowly drawn back into the abdomen and stored in the brood pouch. After the retraction of the ootheca, Chopard says, the ovipositor is completely reversed in position, the prongs being turned forward instead of posteriorly. It would appear, therefore, that in Gomphadorhina the ovipositor withdraws the fully formed ootheca and inserts it into the brood chamber. On the other hand, in the viviparous Diploptera dytiscoides, Hagan (1951) says the eggs “are directed by the ovipositor from the genital chamber ventrally into the open end of the uterus.” In his chapter on Diploptera, Hagan gives a full account of the female reproductive organs and the embryonic development of this viviparous cockroach, with a special description of the pleuropodia and adenopodia and a discussion of the possible functions of these abdominal outgrowths.
Explanation of Lettering on Figures 74–88
a, posterior articulation of mandible, or pleural articulation of coxa.
a’, cranial articulation of maxilla. acgldO, orifice of accessory genital glands.
AcGlds, accessory genital glands, acs, antecostal sulcus.
adhf, adductor muscle of hypopharynx.
admd, adductor muscle of mandible.
admx, adductor muscle of maxilla. af, antennifer.
Amp, ampulla of vas deferens.
An, anus.
ANP, anterior notal wing process.
Ant, antenna.
apl, anapleurite.
Ar, arolium.
AS, antestemite.
at, anterior tentorial pit.
AT, anterior arm of tentorium.
Atr, atrium of spiracle.
Ax, axillary sclerite of wing base (1Ax, 2Ax, 3Ax, first, second, and third axillary).
Ba, basalare, anterior epipleurite.
bcx, basicoxite.
c, anterior articulation of mandible, or trochantinal articulation of coxa.
C, costa, first vein of wing.
Cb, cibarium.
Cd, cardo.
Cer, cercus.
cf, caudal filament.
Clp, clypeus.
cpl, catapleurite, coxapleurite.
CT, central plate of tentorium, corpotentorium.
Cu, cubitus, fifth vein of wing.
Cvx, cervix, neck.
Cx, coxa.
d, alar arm of metascutellum.
Dac, dactyl, median claw of pretarsus.
Dej, ductus ejaculatorius.
df, dorsal fold.
dlcb, dilator muscles of cibarium.
dlmcl, dilator muscle of spiracle.
DT, dorsal arm of tentorium.
e, intertergal sclerites of thorax.
E, compound eye.
Epm, epimeron.
Eppt, epiproct.
eps, episternal apotome.
Eps, episternum.
es, epistomal sulcus.
flcc, cranial flexor muscle of lacinia.
Fm, femur.
For, neck foramen of head, occipital foramen.
Fr, frons.
FrGng, frontal ganglion.
Ga, galea.
Ge, gena.
GC, genital chamber.
Gl, glossa.
glO, orifice of phallic gland.
gnP, gnathal pouch.
Gon, gonapophysis (1Gon, 2Gon, first and second gonapophysis).
Gpr, gonopore.
gr, postocular groove.
h, hinge of lacinia on stipes.
hf, labial fulcrum of hypopharynx.
HP, humeral plate of wing base.
Hphy, hypopharynx.
HS, hypopharyngeal suspensorium.
Hypt, hypoproct.
imB, intermaxillary brachium.
IS, intersternite, spinistemite.
jf, jugal fold of wing.
Ju, jugal region of wing.
L, leg.
Lb, labium.
Lc, lacinia.
lcvpls, lateral cervical plates.
Lg, intermandibular, or interbrachial, ligament.
Lin, lingua.
Lm, labrum.
lPhm, left phallomere.
m, median plate of wing base.
M, media, fourth vein of wing.
mcl, muscle.
Md, mandible.
Mt, mentum.
Mth, mouth.
Mx, maxilla.
o, anterior coxotrochanteral articulation.
Oc, occiput.
occ, occipital condyle.
ocmcl, occlusor muscle of spiracle.
Odl, lateral oviduct.
opl, opposing ventral plates of right phallomere.
otC, oothecal chamber.
Oth, ootheca.
otL, oothecal lobe.
Ovd, oviduct.
Ovp, ovipositor.
p, auxiliary sclerites of unguitractor plate.
Papt, paraproct.
Pcu, postcubital veins of wing.
Pen, penis.
Pge, postgena.
Pgl, paraglossa.
Ph, phragma.
phGld, phallic gland.
phL, primary phallic lobe.
Phm, phallomere.
Phy, pharynx.
Plp, palpus.
PlR, pleural ridge.
PlS, pleural sulcus.
Pmt, postmentum.
PNP, posterior notal wing process.
Poc, postocciput.
PoR, postoccipital ridge.
pos, postoccipital sulcus.
PrC, preoral food cavity.
Prmt, prementum.
Prsct, prescutum.
PS, poststemite.
pt, posterior tentorial pit.
Ptar, pretarsus.
q, euplantulae of tarsus.
r, anterior femorotibial articulation.
R, radius, third vein of wing.
Rect, rectum.
Rm, remigium.
rPhm, right phallomere.
S, sternum.
Sa, subalare, posterior epipleurite.
sAp, sternal apodeme.
Sc, subcosta, second vein of wing.
Scl, scutellum.
Sct, scutum.
Sit, sitophore.
SlDct, salivary duct.
Slin, superlingua.
SlO, orifice of salivary duct.
Slv, salivarium.
Smt, submentum.
Sp, spiracle.
Spt, spermatheca.
sptO, orifice of spermatheca.
St, stipes.
Sty, stylus.
t, tendon.
T, tergum.
Tar, tarsus.
Tb, tibia.
TB, tentorial bridge.
tn, trochantinal apotome.
Tn, trochantin.
Tnt, tentorium.
Tor, torma.
Tr, trochanter.
Tra, trachea.
Un, unguis, lateral claw of pretarsus.
Utr, unguitractor plate.
Vd, vas deferens.
vf, vannal fold of wing.
Vl, valvula of ovipositor (1VI, 2Vl, 3Vl, first, second, and third valvula).
Vn, vannus, fan of wing.
vPhm, ventral phallomere.
vr, ventral ridge of tentorium.
Vs, eversible vesicle of abdomen.
Vsm, vesiculae seminales.
vw, ventral wall of oothecal chamber.
Vx, vertex.
WP, pleural wing process.
x, loral arm of hypopharyngeal suspensorium.
y, oral arm of hypopharyngeal suspensorium.